Direct detection of Ehrlichia canis by PCR in the conjunctiva of a dog with bilateral anterior uveitis-abstract
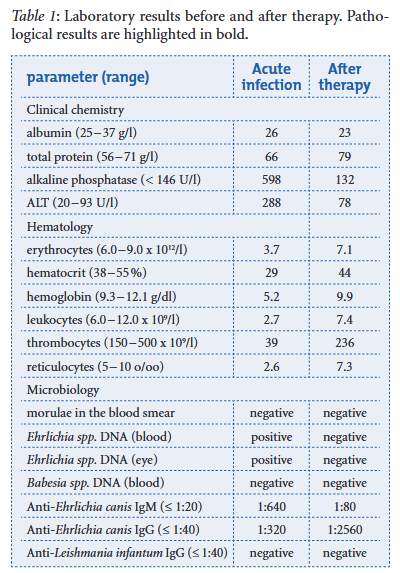
L Walser-Reinhard, D Schaarschmidt-Kiener, J L Forster, F Matheis, B Spiess DOI: 10.1024/0036-7281/a000318 A one-year-old Maltese dog imported from Brazil was presented because of anorexia, dehydration, fever and palpable mandibular lymph nodes. The dog developed clinical signs of bilateral blepharospasm, photophobia and anterior uveitis a few days later. Monocytic ehrlichiosis was diagnosed by positive PCR results from both EDTA blood and conjunctival samples. In addition, Ehrlichia canis-specific IgM and IgG antibodies were both detected. One week after starting treatment with systemic doxycycline and local anti-inflammatory and cycloplegic therapy the dog recovered from systemic and eye diseases. After therapy the follow-up examination revealed a full remission of clinical and hematological parameters and a negative PCR result.
The Thyroid Stimulating Hormone, TSH: Review in Companion Animal
Maigan Espinili Maruquin The serum thyrotropin (thyroid stimulating hormone, TSH) concentration is usually measured in human patients as part of the first-line test for the thyroid function and thus considered best for screening overt and subclinical hyperthyroidism (Ross, Ardisson et al. 1989, Bahn Chair, Burch et al. 2011, Peterson, Guterl et al. 2015). With regards to canine and feline, TSH concentration plays an important role as a biomarker to detect failure of thyroid function, as well. TSH concentration in Canine The hypothyroidism is known to be a common endocrinopathy in dogs. Although it was believed that some cases of canine hypothyroidism were caused by autoimmune thyroiditis, it may also be a result from an inability of the pituitary gland to synthesize and secrete TSH, which results to secondary thyroid follicular atrophy (Ferguson 2007). A classic case of adult-onset hypothyroidism present symptoms of lethargy, weakness, dullness of mental attitude and dermatological problems including alopecia or recurrent skin infections however, in some cases, there are only manifestations of general neuropathy or myopathy (Jaggy and Oliver 1994, Jaggy, Oliver et al. 1994, Ferguson 2007). Being considered as a multisystemic disorder, general medical evaluation should be conducted for suspected cases, including clinicopathologic evaluations (Ferguson DC, Hoenig ME, 1991; Ferguson DC, Hoenig M., 2003) (Ferguson 2007). https://www.horshamvethospital.com.au/hypothyroidism-in-dogs Fig. 01. A canine manifesting signs of hypothyroidism Having false-positive test results are common in hypothyroidism in canines and diagnosis can be challenging, thus, diagnostic testing should be considered to animals presenting clinical and clinicopathologic signs that are consistent with hypothyroidism (McCann 2015). The mostly used diagnostic criteria is with low total or free T4 concentration combined with a high TSH concentration in serum (Dixon and Mooney 1999, Shiel, Sist et al. 2010). The elevated concentration of TSH in dogs with primary hypothyroidism is expected in response to negative feedback of thyroid hormones on the pituitary gland. However, TSH as a sole marker of the disease is not effective due to possibilities of normal TSH in hypothyroid dogs and elevated TSH in euthyroid dogs, resulting to poor sensitivity. Thus, it is usually combined with reduced T4 or free T4 for higher specificity in diagnosis (McCann 2015). TSH concentration in Feline The hyperthyroidism in cats is a common disease in older cats and like in humans, hyperplastic or adenomatous nodular changes in the thyroid are present and peak at the old age (Wakeling, Elliott et al. 2011). This common feline endocrine disease has multiple factors likely involved, including genetic susceptibility (Taylor 2017) and has been diagnosed in 1.5–11.4% of older cats across the world (Carney, Ward et al. 2016, Taylor 2017). Felines with this disorder present signs that vary from the described as classic cat with weight loss, polyphagia, polyuria, polydipsia, increased vocalization, agitation, increased activity, tachypnea, tachycardia, vomiting, diarrhea and an unkempt hair coat, lack of appetite, and lethargy to severely hypermetabolic clinical presentation (Carney, Ward et al. 2016, Taylor 2017). Important comorbidities recorded were cardiorespiratory diseases (Fox, Peterson et al. 1999). A commercial feline-specific thyroid-stimulating hormone (TSH) assay is not yet available in the market, and in some cases, feline samples are analyzed using canine TSH assays, however, delicate differences in TSH at low levels may evade detection. The TSH should always be interpreted with corresponding tT4 or free T4 results (Taylor 2017). An excellent sensitivity is highly suggested if the TSH below the limit of detection is found in a hyperthyroid cat (Peterson ME, 2016)(Taylor 2017). References: Ferguson DC, Hoenig ME. Canine hypothyroidism. In: Allen DG, editor. Small animal medicine. Philadelphia: J.B.Lippincott Co; 1991. p. 845–65. Ferguson DC, Hoenig M. Endocrine system. In: Latimer KS, Mahaffey EA, Prasse KW, editors. Duncan and Prasse’s veterinary laboratory medicine: clinical pathology. 4th edition. Ames (IA): Iowa State Press; 2003. p. 270–303 Peterson ME (2016) Diagnosis and management of iatrogenic hypothyroidism. In: Little SE, ed. August’s Consultations in Feline Medicine Volume 7. Elsevier, St Louis, MO: 260–9 Bahn Chair, R. S., H. B. Burch, D. S. Cooper, J. R. Garber, M. C. Greenlee, I. Klein, P. Laurberg, I. R. McDougall, V. M. Montori, S. A. Rivkees, D. S. Ross, J. A. Sosa and M. N. Stan (2011). “Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists.” Thyroid 21(6): 593-646. Carney, H. C., C. R. Ward, S. J. Bailey, D. Bruyette, S. Dennis, D. Ferguson, A. Hinc and A. R. Rucinsky (2016). “2016 AAFP Guidelines for the Management of Feline Hyperthyroidism.” Journal of Feline Medicine and Surgery 18(5): 400-416. Dixon, R. M. and C. T. Mooney (1999). “Evaluation of serum free thyroxine and thyrotropin concentrations in the diagnosis of canine hypothyroidism.” J Small Anim Pract 40(2): 72-78. Ferguson, D. C. (2007). “Testing for hypothyroidism in dogs.” Vet Clin North Am Small Anim Pract 37(4): 647-669, v. Fox, P. R., M. E. Peterson and J. D. Broussard (1999). “Electrocardiographic and radiographic changes in cats with hyperthyroidism: comparison of populations evaluated during 1992-1993 vs. 1979-1982.” J Am Anim Hosp Assoc 35(1): 27-31. Jaggy, A. and J. E. Oliver (1994). “Neurologic manifestations of thyroid disease.” Vet Clin North Am Small Anim Pract 24(3): 487-494. Jaggy, A., J. E. Oliver, D. C. Ferguson, E. A. Mahaffey and T. G. Jun (1994). “Neurological Manifestations of Hypothyroidism: A Retrospective Study of 29 Dogs.” Journal of Veterinary Internal Medicine 8(5): 328-336. McCann, T. (2015). “Canine hypothyroidism.” Companion Animal 20(10): 572-578. Peterson, M. E., J. N. Guterl, R. Nichols and M. Rishniw (2015). “Evaluation of Serum Thyroid-Stimulating Hormone Concentration as a Diagnostic Test for Hyperthyroidism in Cats.” Journal of Veterinary Internal Medicine 29(5): 1327-1334. Ross, D. S., L. J. Ardisson and M. J. Meskell (1989). “Measurement of thyrotropin in clinical and subclinical hyperthyroidism using a new chemiluminescent assay.” J Clin Endocrinol Metab 69(3): 684-688. Shiel, R. E., M. Sist, R. F. Nachreiner, C. P. Ehrlich and C. T. Mooney (2010). “Assessment of criteria used by veterinary practitioners to diagnose hypothyroidism in sighthounds and investigation of serum thyroid hormone concentrations in healthy Salukis.” Journal of the American Veterinary
Leptospirosis and immune-mediated hemolytic anemia: A lethal association
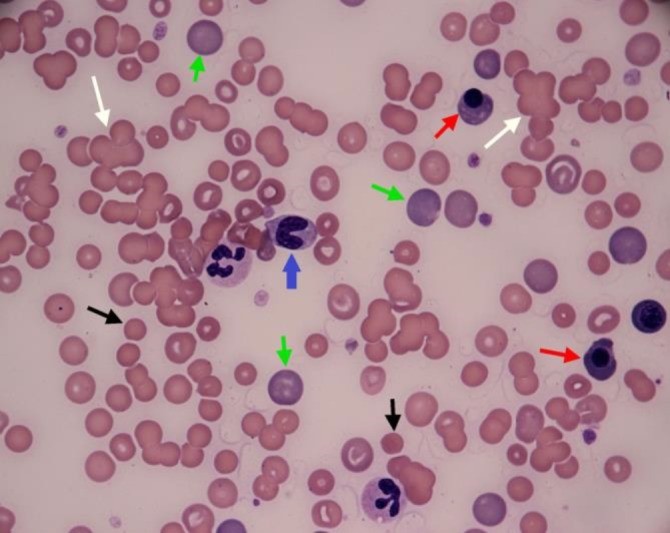
Tommaso Furlanello* and Ida Reale Vet Res Forum. 2019 Summer; 10(3): 261–265. Published online 2019 Sep 15. doi: 10.30466/vrf.2019.99876.2385 An eight-year old crossbreed dog was referred to San Marco Veterinary Clinic with a history of acute illness presenting a severe hemolytic anemia, and intense icterus most likely immune-mediated condition. Circulating antibodies against red blood cells were detected via flow cytometry, while vector-borne diseases were ruled out because of negative results of serological tests for the most common vector-borne diseases in this case. However, leptospirosis was excluded. This resulted in an unsuccessful immunosuppressive therapy with prednisone, two whole blood transfusions and ultimately death of the patient. Leptospirosis was confirmed by both micro-agglutination test for antibodies and PCR test of urine sample. Fig. 1 Blood Smear of the examined dog. White arrows: agglutinates, green arrows: large polycromatophils RBCs, black arrows: spherocytes; red arrow: nucleated RBC, blue arrow: band granulocyte neutrophil. The two-segmented neutrophils visible in the picture display foamy and basophilic cytoplasm, as signs of toxicity, (Diff Quik stain; 100×).
The Importance of AGP in FIP Diagnosis: An Overview
Maigan Espinili Maruquin The Feline Infectious Peritonitis (FIP) The coronaviruses are enveloped, positive-sense single-stranded RNA viruses with non-segmented genomes of around 30,000 nucleotides in length (Tasker 2018)( Siddell SG, 1995). The feline coronavirus (FCoV) has two pathotypes distinguished by their biological behavior. The highly prevalent feline enteric coronavirus (FECV) is highly contagious with transmission from faeces of shedding cats (Felten and Hartmann 2019). However, most cases are asymptomatic or displays mild gastrointestinal clinical signs, (Addie, Toth et al. 1995, Pedersen, Sato et al. 2004, Pedersen, Allen et al. 2008, Pedersen 2009, Vogel, Van der Lubben et al. 2010, Tasker 2018, Felten and Hartmann 2019). On the other hand, the feline infectious peritonitis virus (FIPV) is a mutation within a small percentage of infected cats and it results to a fatal disease feline infectious peritonitis (FIP), commonly in young cats (Pedersen, Boyle et al. 1981, Pedersen, Boyle et al. 1981, Addie, Toth et al. 1995, Vennema, Poland et al. 1998, Pedersen 2009, Tasker 2018, Felten and Hartmann 2019). However, the exact gene causing mutation is still unknown (Felten and Hartmann 2019). The FIP may appear in two clinically distinct forms: the wet form and dry form, which is effusive and granulomatous forms, respectively (Wolfe and Griesemer 1966, Montali and Strandberg 1972, Pedersen 2009, Hazuchova, Held et al. 2016). The development of FIP is affected by three factors. First is the viral factor wherein studies relative to mutation of the FCoV S gene where presented (Tasker 2018) and the replication in monocytes, and activation of infected monocytes were also considered important in the development of FIP (Kipar and Meli 2014, Tasker 2018). Second factor considered is the host’s immune response, breed and genetic (de Groot-Mijnes, van Dun et al. 2005, Dewerchin, Cornelissen et al. 2005, Golovko, Lyons et al. 2013, Pedersen, Liu et al. 2016, Tasker 2018). Finally, another factor affecting the FIP development is the environment- level of stress and overcrowding (Tasker 2018). https://www.researchgate.net/figure/Cat-with-wet-effusive-form-of-FIP-presenting-moderate-abdominal-distention-due-to_fig7_51758582 Fig. 01. Manifestation of moderate abdominal distention due to peritoneal effusion. A clinical sign of wet (effusive) FIP. Common clinical signs for the FIP infected cats include lethargy, anorexia, weight loss, fluctuating pyrexia, and sometimes presents jaundice (Tasker 2018). On the other hand, wet FIP cases can be associated with abdominal, pleural and/ or pericardial effusions, and progresses within few days to weeks with severe limiting survival (Ritz, Egberink et al. 2007, Tasker 2018). Whereas, dry FIP usually displays neurological signs (Crawford, Stoll et al. 2017) or ocular signs, which progresses in a few weeks to months and are more chronic (Tasker 2018). The alpha- 1 acid glycoprotein (AGP) in FIP diagnosis Prior to acquired immune response, part of the innate response is the acute phase response (Murata, Shimada et al. 2004, Schmidt and Eckersall 2015). Proteins known as the acute phase proteins (APPs) are then increased in production from hepatocytes and peripheral tissues and then released (Schmidt and Eckersall 2015). These blood proteins can be used to evaluate the innate response to infection, inflammation or trauma (Murata, Shimada et al. 2004, Petersen, Nielsen et al. 2004, Ceron, Eckersall et al. 2005, Eckersall and Bell 2010). With changes by >25% in the serum concentration in response to disease stimulation, APPs are considered useful quantitative biomarkers of diseases- in diagnosis, prognosis, response to therapy, and in general health screening (Eckersall and Bell 2010). In response to inflammations, the serum alpha- 1 acid glycoprotein (AGP) concentration increases as a major acute phase protein in cats (Ceron, Eckersall et al. 2005, Paltrinieri 2008, Giori, Giordano et al. 2011). Studies showed increased serum AGP concentration in cats infected with FIP (Duthie, Eckersall et al. 1997, Giordano, Spagnolo et al. 2004, Giori, Giordano et al. 2011). The feline AGP in both serum and peritoneal fluid are known biomarker for FIP (Duthie, Eckersall et al. 1997, Giordano, Spagnolo et al. 2004, Eckersall and Bell 2010). In a study conducted, AGP in effusion showed to be the best APP to distinguish between cats with and without FIP (Hazuchova, Held et al. 2016). Although AGP elevations are not specific for FIP, the measurement is helpful in the diagnosis of FIP, and levels >1.5 mg/ml are often observed in FIP cases (Tasker 2018). It was then concluded that the higher levels increase the index of suspicion (Duthie, Eckersall et al. 1997, Paltrinieri, Giordano et al. 2007, Giori, Giordano et al. 2011, Hazuchova, Held et al. 2016, Tasker 2018). With difficulty in diagnosing FIP through conventional approaches (Addie, Paltrinieri et al. 2004, Paltrinieri 2008), samples from FIP infected cats showed AGP seemed to be associated with viral antigen and are seen present in large amounts (Paltrinieri, Giordano et al. 2004, Paltrinieri 2008). Nevertheless, AGP plays role in drug-binding, as an immunomodulatory agent, and acts as a plasma transport protein (Ceron, Eckersall et al. 2005, Ceciliani, Ceron et al. 2012, Schmidt and Eckersall 2015). References: Siddell SG. The coronaviridae. London: Plenum Press, 1995 Drechsler, Y., Alcaraz, A., Bossong, F., Collisson, E.W., Diniz, P. (2011), “Feline Coronavirus in Multicat Environments”. Veterinary Clinics of North America Small Animal Practice 41(6):1133-69. Addie, D. D., S. Paltrinieri, N. C. Pedersen and s. Secong international feline coronavirus/feline infectious peritonitis (2004). “Recommendations from workshops of the second international feline coronavirus/feline infectious peritonitis symposium.” Journal of feline medicine and surgery 6(2): 125-130. Addie, D. D., S. Toth, G. D. Murray and O. Jarrett (1995). “Risk of feline infectious peritonitis in cats naturally infected with feline coronavirus.” Am J Vet Res 56(4): 429-434. Ceciliani, F., J. J. Ceron, P. D. Eckersall and H. Sauerwein (2012). “Acute phase proteins in ruminants.” J Proteomics 75(14): 4207-4231. Ceron, J. J., P. D. Eckersall and S. Martýnez-Subiela (2005). “Acute phase proteins in dogs and cats: current knowledge and future perspectives.” Vet Clin Pathol 34(2): 85-99. Crawford, A. H., A. L. Stoll, D. Sanchez-Masian, A. Shea, J. Michaels, A. R. Fraser and E. Beltran (2017). “Clinicopathologic Features and Magnetic Resonance Imaging Findings in 24 Cats With Histopathologically Confirmed Neurologic Feline Infectious Peritonitis.” J Vet Intern Med 31(5): 1477-1486.
Feline NT- proBNP as a cardiac biomarker
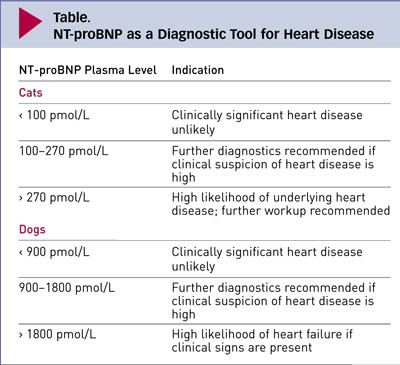
Maigan Espinili Maruquin Feline NT- proBNP The atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are hormones released into the circulation in response to stimuli. These Natriuretic peptides (NP) are synthesized by cardiomyocytes which regulates body fluid homeostasis and blood pressure (Wilkins, Redondo et al. 1997, Connolly 2010)( Martinez RA, et al., 2009). From preprohormones, NP are processed to prohormones (Blake 2018). The proANP and proBNP, once released, quickly cleaves into separate inactive N-terminal (NT-proANP, NT-proBNP) and active C-terminal (C-ANP, C-BNP) fragments (Oyama 2013, Blake 2018). In humans, ANP has shorter half- life than the BNP (Suga, Nakao et al. 1992, Blake 2018). On the other hand, while C-terminal provides counterbalance to those of the renin-angiotensin-aldosterone system, it has shorter half-life than that of the N-terminal, making NT-proBNP more stable for assay detection (Potter 2011, Oyama 2013, Blake 2018). Hypertrophic Cardiomyopathy (HCM) The HCM has a prevalence of around 15% among cats wherein this disease of the myocardium causes abnormal thickening of the walls of the left ventricle (LV) (Paige, Abbott et al. 2009, Abbott 2010, Wagner, Fuentes et al. 2010, Payne, Brodbelt et al. 2015, Luis Fuentes and Wilkie 2017). Some cats experiencing HCM develop congestive heart failure (CHF), arterial thromboembolism (ATE), or sudden cardiac death (SCD) (Payne, Borgeat et al. 2013, Payne, Borgeat et al. 2015, Luis Fuentes and Wilkie 2017). Usually, HCM in felines is detected incidentally on routine veterinary examinations through auscultatory findings including arrhythmias, gallop sounds, or murmurs, while sometimes, detection is from heart failure clinical signs or embolism (Atkins, Gallo et al. 1992, Rush, Freeman et al. 2002, Abbott 2010). Moreover, HCM is considered the most prevalent myocardial disorder in cats (Fox, Liu et al. 1995, Fox, Basso et al. 2007, Paige, Abbott et al. 2009, Fox, Rush et al. 2011). Due to limited sensitivity and specificity of physical examination, electrocardiography (ECG) and thoracic radiography, diagnosing cardiomyopathy has been a challenge (Côté, Manning et al. 2004, Wood and Picard 2004, Schober, Maerz et al. 2007, Harris, Estrada et al. 2017), whereas, the current clinical gold standard used in cats is echocardiography (Harris, Estrada et al. 2017). Although echocardiography has high specificity in diagnosing myocardial disease (Wood and Picard 2004, Fox, Rush et al. 2011), its sensitivity is sometimes limited in detecting HCM (Fox, Rush et al. 2011). The efficacy of treatments for the HCM has limited knowledge. Some agents were suspected to slow the progression of HCM in other breeds of cats, considering the probable existence of genetic heterogeneity in feline HCM. Interventions to speed myocardial relaxation or slow heart rate were also observed in attempt to improve diastolic function. (Abbott 2010). Feline NT- proBNP Assay Table 1. Plasma Levels of Feline NT-proBNP and their indications (https://www.cliniciansbrief.com/article/cardiac-n-terminal-pro-b-type-natriuretic-peptide-assay) There is an increase of interest in the usefulness of cardiac biomarker measurement in veterinary practice (Oyama 2013). In cats, there are available, inexpensive, not requiring advance training cardiac biomarkers for initial screening cardiomyopathy (Luis Fuentes and Wilkie 2017)( Charron P., et al., 2003). The echocardiography has a huge role in feline diagnosis of structural heart disease, however, due to its availability and appropriateness in in emergent circumstances, the use of NT-proBNP assays have been stimulated in differentiating CHF and non- cardiac causes of respiratory distress (Oyama, Boswood et al. 2013). A biomarker is considered clinically useful if it provides information on diagnosis, prognosis, or response to treatment (Oyama 2013). The NT-proBNP in cats can be measured by conducting feline-specific NT-proBNP assay (Singletary, Rush et al. 2012). It has been reported that low NT-proBNP concentration is mostly non- cardiac cause (Oyama 2013) while cats with increased NT-proBNP were reported to have clinically relevant structural heart and elevated concentration suggests CHF (Connolly, Magalhaes et al. 2008, Fox, Oyama et al. 2009, Fox, Rush et al. 2011, Singletary, Rush et al. 2012, Oyama 2013). The NT-proBNP test appears to be not useful for breeding examination (Hsu, Kittleson et al. 2009, Singh, Cocchiaro et al. 2010, Hassdenteufel, Henrich et al. 2013). References: Martinez-Rumayor A, Richards AM, Burnett JC, et al. Biology of the natriuretic peptides. Am J Cardiol 2009; 101:3–8. Charron P, Forissier JF, Amara ME, et al. Accuracy of European diagnostic criteria for familial hypertrophic cardiomyopathy in a genotyped population. Int J Cardiol 2003; 90:33–38 Abbott, J. A. (2010). “Feline Hypertrophic Cardiomyopathy: An Update.” Veterinary Clinics: Small Animal Practice 40(4): 685-700. Atkins, C. E., A. M. Gallo, I. D. Kurzman and P. Cowen (1992). “Risk factors, clinical signs, and survival in cats with a clinical diagnosis of idiopathic hypertrophic cardiomyopathy: 74 cases (1985-1989).” J Am Vet Med Assoc 201(4): 613-618. Blake, R. (2018). “The use of cardiac biomarkers in dogs and cats.” Companion Animal 23(10): 569-577. Connolly, D. J. (2010). “Natriuretic Peptides: The Feline Experience.” Veterinary Clinics: Small Animal Practice 40(4): 559-570. Connolly, D. J., R. J. Magalhaes, H. M. Syme, A. Boswood, V. L. Fuentes, L. Chu and M. Metcalf (2008). “Circulating natriuretic peptides in cats with heart disease.” J Vet Intern Med 22(1): 96-105. Côté, E., A. M. Manning, D. Emerson, N. J. Laste, R. L. Malakoff and N. K. Harpster (2004). “Assessment of the prevalence of heart murmurs in overtly healthy cats.” J Am Vet Med Assoc 225(3): 384-388. Fox, P. R., C. Basso, G. Thiene and B. J. Maron (2007). Spontaneous Animal Models. Arrhythmogenic RV Cardiomyopathy/Dysplasia: Recent Advances. F. I. Markus, A. Nava and G. Thiene. Milano, Springer Milan: 69-78. Fox, P. R., S. K. Liu and B. J. Maron (1995). “Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. An animal model of human disease.” Circulation 92(9): 2645-2651. Fox, P. R., M. A. Oyama, C. Reynolds, J. E. Rush, T. C. DeFrancesco, B. W. Keene, C. E. Atkins, K. A. Macdonald, K. E. Schober, J. D. Bonagura, R. L. Stepien, H. B. Kellihan, T. P. Nguyenba, L. B. Lehmkuhl, B. K. Lefbom, N. S. Moise and D. F. Hogan (2009). “Utility of plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) to distinguish between congestive heart failure and non-cardiac
Urinary tract infection caused by methicillin-resistant Staphylococcus pseudintermedius in a dog
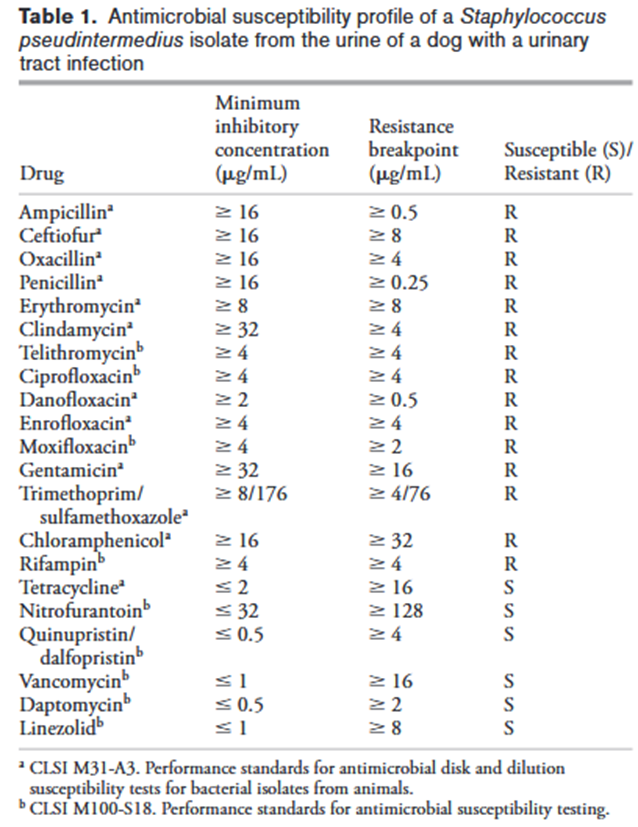
Joseph E. Rubin and Matthew C. Gaunt Source: Can Vet J. 2011 Feb; 52(2): 162–164. A 16-month-old neutered male pug dog was presented on emergency due to hematuria and pollakiuria of 2-days duration. A urinary tract infection caused by methicillin-resistant Staphylococcus pseudintermedius (MRSP) was diagnosed, following culture and susceptibility testing. The isolated MRSP was susceptible to only tetracycline among commonly used antimicrobials. Treatment with doxycycline led to bacteriological cure and resolution of clinical signs. No presumptive risk factors for acquisition of MRSP were identified in this case indicating that the infection was community acquired.
KNOWING THE THREAT OF CANINE BABESIOSIS
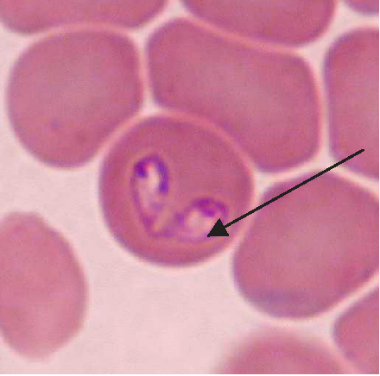
Maigan Espinili Maruquin Our pets are members of our family. And like the other members, they need care, love, and attention. We spend most of our time in the work, school, and other daily activities we have, which lessens the time we spend with our pets. However, we should also listen to our companions who stays at home. Attend to their needs, specially their health. As important as the humans, dogs also need regular medical checks to make sure that they are living a happy and healthy life. One of the diseases you should be cautious of when you have a dog companion are those caused by parasites. For both domestic and wild canines, canine babesiosis is an important widespread disease. The disease is caused by single-celled microorganisms (protozoa) belonging to the Babesia family. Babesia parasites are primarily spread by the bite of an infected tick. The distribution of the canine Babesia species are greatly affected by the presence of tick vector species in the area. Babesia infecting dogs are morphologically classified into forms of large, including B. canis, B. volgeli, B. rossi, and small, including B. gibsoni, both exhibiting a worldwide distribution. Among Babeisa species infecting dogs, B. gibsoni has been recognized as an important pathogen that affects dogs in the Middle East, Africa, Asia, Europe, and many areas of the United States. The babesiosis is associated mainly in haemolytic anaemia or the destruction and breaking down of the red blood cells. B. gibsoni can cause hyperacute, acute, and chronic infections. Clinical signs present are ambiguous, which includes depression, lethargy, fever, weakness, vomiting, pale gums and anorexia. However, other specific signs include dark coloration of the urine, neurological dysfunction, respiratory failure, jaundice, and sometimes presence of bleeding diatheses. Severe cases may lead to organ failure and death. However, some cases during the initial stages appear to be unnoticed to the pet owners. On the other hand, chronic stages often make the dog a carrier of the organism and becomes asymptomatic, and for how long will a dog be a carrier is unknown. How do dogs get infected with babesiosis? The Babesia species resides in its first host, the tick vector. Dogs become infected when ticks feed for 2 to 3 days and release sporozoites, from the salivary gland, into the circulation of dogs. Inside the host, sporozoites start to invade red blood cells, and replicate via binary fission, which produces merozoites to further invade other red blood cells. Ticks become infected with merozoites during feeding, and sexual reproduction of Babesia’s life cycle completes within the ticks. In addition, transmission can also occur through transfusion of infected blood, transplacental transmission (to unborn puppies in the uterus of their mothers), or direct blood-blood contact during fighting. How is babesiosis diagnosed? Canine babesiosis is historically identified based on the observation of the parasite within red blood cells using light microscope. Infection of large or small form of Babesia can be morphagically identified, if enough parasites present in the blood, in the blood smear. Other diagnostic tests detecting antigen include FA (fluorescent antibody) staining of the organism and PCR (polymerase chain reaction) detecting nucleic acid of the Babesia. The PCR test has the advantage in that it can identify all four species of Babesia, but requires trained persons to run it. Serologic or antibody testing may also be performed to see the presence of the parasite. Antibody reaction to the Babesia infection can be measured by ELISA (enzyme-linked immunosorbent assay) in the lab. In addition, a lateral flow immunochromatographic test (or rapid test) has been developed to provide a fast and in-site assay to detect the antibody of dogs infected with B. gibsoni. This rapid test has been commonly assisting the veterinarians for fast diagnosis. Disease Management and Prevention The treatment of an infected dog consists of three components: antiprotozoal treatment of babesiosis, blood transfusions to treat severe anaemia, and supportive therapies for the complications and metabolic derangements. Meanwhile, as a pet owner, regular control of the tick vectors by routinely dipping or spraying pets or using tick collars or spot-on preparations is the only effective way of preventing this disease in most parts of the world. Ticks,if founed on your pets, shouldn’t be squeezed, crushed or twisted to avoid the parasite from being expelled. Removing it properly shall be done or ask the assistance of your veterinarians. To take the ticks off your dog, tick’s mouth should be grasped as close to the skin as possible using forceps. Tick’s mouth shall be removed as much as possible. Afterwhich, make sure to clean the tick bitten area with soap and water or using mild antibacterial wound cleanser. In addition, preventing dogfighting as well as direct blood contact by using sterilized instruments during tail docking and ear cropping procedures and when administering injections are critical. Moreover, a vaccine against B. canis has become commercially available in some countries. You may check with your pet’s veterinarians prior to visiting endemic areas with your dogs. Fig. 1. Two pear-shaped Babesia canis organisms in an erythrocyte. (Duh et al., 2004) Fig. 2. Babesia gibsoni in erythrocytes in a blood smear stained with modified Wright technique. (Trotta et al., 2009) Chauvin A., Moreau E., Bonnet S., Plantard O. & Malandrin L. Babesia and its hosts: adaptation to long-lasting interactions as a way to achieve efficient transmission. 2009. Vet. Res., 40 (2), 37.64. Duh D, Tozon N, Petrovec M, Strasek K, Avsic-Zupanc T. Canine babesiosis in Slovenia: molecular evidence of Babesia canis canis and Babesia canis vogeli. 2004. Vet Res. 35(3):363-8. Refer Conrad P., Thomford J., Yamane I., Whiting J., Bosma L., Uno T., Holshuh H.J. & Shelly S. Hemolytic anemia caused by Babesiagibsoni infection in dogs. 1991. J. Am. Vet. Med. Assoc., 199 (5): 601–605. Trotta M, Carli E, Novari G, Furlanello T, Solano-Gallego L. Clinicopathological findings, molecular detection and characterization of Babesia gibsoni infection in a sick dog from Italy. 2009. Vet Parasitol. 165(3-4):318-22.
Case report: systemic tuberculosis caused by Mycobacterium bovis in a cat- Abstract
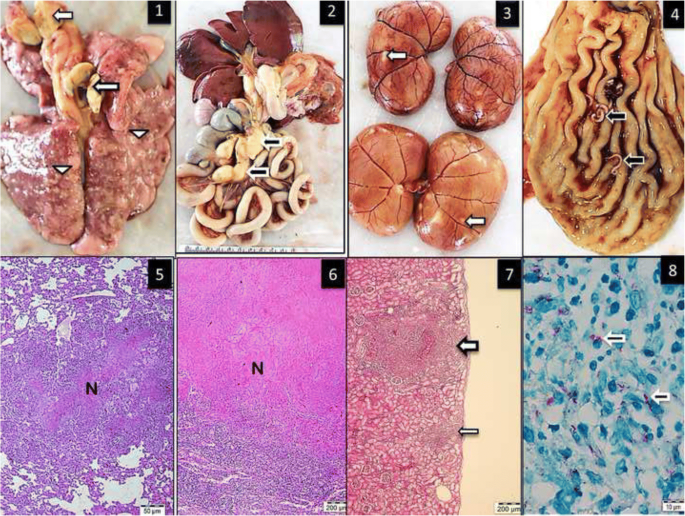
Source: https://bmcvetres.biomedcentral.com/articles/10.1186/s12917-018-1759-7 Mycobacterium bovis was isolated from the lungs, bronchial and gastrointestinal lymph nodes, kidney and liver of a 5-year-old stray male cat. The isolate was confirmed as M. bovis using the Genotype MTBC assay (Hain Lifescience, Germany), which allows differentiation of species within the Mycobacterium tuberculosis complex. The Systemic tuberculosis was diagnosed via postmortem examination of the cat. Pathological changes included multifocal to coalescing granulomatous inflammation in the lungs, liver, lymph nodes and kidneys. Infection by immunosuppressive viral pathogens including feline herpes virus-1, feline immunodeficiency virus and feline parvovirus virus were ruled out by polymerase chain reaction assay (PCR). The isolated M. bovis was susceptible to isoniazid, ethambutol, rifampicin or streptomycin. Unlike previous cases of feline tuberculosis in Turkey, this case report details the first case of feline tuberculosis in Turkey for which the causative agent (M. bovis) was confirmed with bacteria isolation, morphological evaluation, molecular characterization and antibiotic sensitivity. Figure Multifocal granulomatous pneumonia (arrowhead) and diffuse lymphadenitis (arrow) of tracheobronchial lymph nodes. 2. Diffuse, severe lymphadenitis in mesenteric lymph nodes (arrow). 3. Multifocal granulomatous nephritis. 4. Gastric worms on the mucosal surface (arrows). 5.6.7. Granulomatous pneumonia (5) with necrosis (N), lymphadenitis (6) and necrosis and nephritis (7) with granulomas (arrows), HE. 8. Acid-fast microorganisms in the cytoplasm of epithelioid macrophages (arrows), ZN
Fulminant Tritrichomonas foetus ‘feline genotype’ infection in a 3-month old kitten associated with viral co-infection
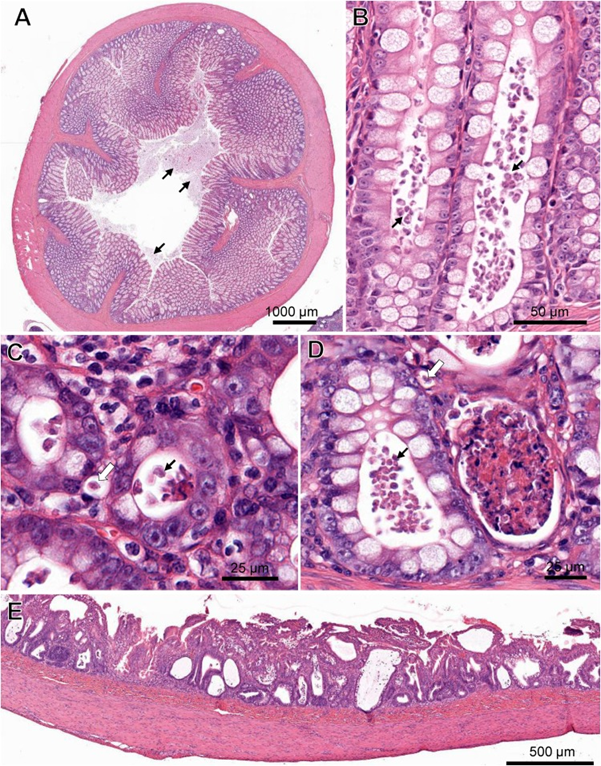
Laura Setyo, Shannon L. Donahoe, and Jan Šlapeta Original article: https://doi.org/10.1016/j.vetpar.2018.12.007 Tritrichomonas foetus is a microscopic single-celled flagellated protozoan parasite which mainly causes colitis (large bowel diarrhea) in young cats and kittens. A 3-month-old Bengal kitten showed severe T. foetus infection of the colon, cecum and ileum with concurrent feline enteric coronavirus (FCoV) and feline panleukopenia virus (FPV). The kitten had an 8-day history of vomiting, diarrhoea, failure to thrive and coughing prior to being presented to the University of Sydney Veterinary Teaching Hospital. Protozoa filling the lumen and crypts and occasional invading into lamina propria were identified within the affected colon and confirmed by PCR as T. foetus‘feline genotype’. FCoV and FPV co-infection were also identified from fecal samples of the kitten via PCR. Immunosuppression caused by FPV may play a role in the unprecedented T. foetus infection intensity observed histologically. Gastrointestinal pathology in a cat co-infected with Tritrichomonas foetus, feline enteric coronavirus (FCoV) and feline panleukopenia virus (FPV). (A) Cross-section of the colon with marked protozoal (T. foetus) luminal infiltrate (arrows). ( B) Numerous teardrop-shaped T. foetus overlying the mucosal luminal surface of a crypt of Lieberkühn in the colon. (C) T. foetus (arrow) in the cross-section of the crypts of the colon. (D) Crypt abscesses containing cellular and karyorrhectic debris and T. foetus (arrows) in the colon. Note the invading T. foetus in the lamina propria (arrow outlines). (E) Severe attenuation of the superficial epithelium of the small intestinal of the cat PCR positive for FCoV and FPV. H&E. (Virtual Slide for Virtual Microscopy: VM05606).
Serum Canine Pancreatic Lipase Immunoreactivity Assay (cPLI) in Diagnosing Canine Pancreatitis
Maigan Espinili Maruquin The lipases are present from a variety of cells, including pancreatic, hepatic, and gastric cells, with similar function, ie , hydrolysis of triglycerides (Xenoulis and Steiner 2012)( Steiner JM, 2000)(Dröes and Tappin 2017). From the pancreatic origin, the canine pancreatic lipase increases in the event of pancreatic inflammation (Steiner and Williams 2003, Haworth, Hosgood et al. 2014). The canine pancreatic lipase immunoreactivity (cPLI) assay was first a radioimmunoassay, and subsequently an enzyme immunoassay, eventually developed into a commercially available specific canine pancreatic lipase assay (Steiner, Teague et al. 2003, Steiner and Williams 2003, Haworth, Hosgood et al . 2014). The serum cPLI is now used as an important specific, and sensitive marker for the exocrine pancreas (Steiner, Newman et al. 2008, Neilson-Carley, Robertson et al. 2011, Trivedi, Marks et al. 2011, Xenoulis and Steiner 2012, Mawby, Whittemore et al. 2014, Dröes and Tappin 2017). Canine Pancreatitis Disorders originating from the liver and pancreas are considered important causes of morbidity and mortality in both dogs and cats, which presents different sets of challenges in diagnosis (Lidbury and Suchodolski 2016). Dogs have several exocrine pancreas diseases including exocrine pancreatic insufficiency (EPI), pancreatic carcinoma, and pancreatitis (Lidbury and Suchodolski 2016). Among them, pancreatitis is the most common disorder and are mostly considered idiopathic (Xenoulis and Steiner 2012). Pancreatitis is the inflammation of the exocrine pancreas, wherein there is an infiltration with inflammatory cells. The term is usually expanded to include other diseases of the exocrine pancreas by necrosis or necrotising pancreatitis, or irreversible structural changes such as fibrosis (chronic pancreatitis) (Xenoulis and Steiner 2012). The pancreatitis is in acute stage when there is neutrophilic inflammation while chronic is characterized by the acinar atrophy and fibrosis (Newman, Steiner et al. 2006, Watson, Roulois et al. 2007, Mansfield, Anderson et al. 2012 , Watson 2015, Dröes and Tappin 2017). https://i2.wp.com/thewholedog.com/wp-content/uploads/2014/04/pancreatiitis.jpg?ssl=1 Fig. 01. An illustration of the difference of healthy pancreas vs. inflamed pancreas in dogs Despite being a common disorder in canines, the antemortem diagnosis of pancreatitis is clinically challenging (Trivedi, Marks et al. 2011). Clinical signs are non-specific and varies greatly. Subclinical diseases are evident in most cases while others may display mild and non -specific clinical signs (Xenoulis 2015) which may include anorexia, vomiting, lethargy, diarrhea, melena, weight loss, hematemesis, and hematochezia (Hess, Saunders et al. 1998, Trivedi, Marks et al. 2011). Dogs with acute pancreatitis are at risk of developing obesity, diabetes mellitus, hyperadrenocortcisim, hypothyroidism, prior gastrointestinal disease, and epilepsy (Cook, Breitschwerdt et al. 1993, Trivedi, Marks et al. 2011). On the other hand, cardiovascular shock,disseminated intravascular coagulation (DIC) or multi-organ failure and death within hours of the development of clinical signs are presented in severe cases (Xenoulis 2015, Dröes and Tappin 2017). As diagnosis of pancreatitis remains a challenge, histopathology is considered the gold standard for diagnosis of pancreatitis. However, with procedural risks and difficulty to determine the right area for biopsy, obtaining the samples is hard (Newman, Steiner et al. 2004, Dröes and Tappin 2017). Therefore, considering the improving sensitivity and specificity of laboratory tests, using a combination of different methods can be best used (Dröes and Tappin 2017). On the other hand, there are treatments for acute pancreatitis infected dogs. Treatments consider Intravenous Fluid Therapy, knowing that pancreatitis disturbs pancreatic microcirculation (Bassi, Kollias et al. 1994, Mansfield 2012). The administration of plasma is claimed to correct hypoalbuminemia, replacement of circulating α-macroglobulins, replacement of coagulation factors, and amelioration of systemic inflammation, however, no controlled studies on plasma transfusion in dogs with naturally occurring acute pancreatitis has proven its benefit (Mansfield 2012). Due to vomiting of infected dogs, anti-emetics are used to manage acute pancreatitis (Mansfield 2012). On the other hand, corticosteroids enhance apoptosis, and increase the production of pancreatitis-associated protein, giving protective effect against pancreatic inflammation (Zhang, Kandil et al. 2004, Mansfield 2012). Moreover, diet should also be managed including the treatment of the complications of the disease (Mansfield 2012) Canine Pancreatic Lipase Immunoreactivity Assay Due to the limitations of the traditional golden standard, histopathology, diagnosis has relied on clinical criteria as an alternative gold standard (Graca, Messick et al. 2005, McCord, Morley et al. 2012, Cridge, MacLeod et al. 2018, Gori, Lippi et al. 2019, Nielsen, Holm et al. 2019, Okanishi, Nagata et al. 2019, Cridge, Mackin et al. 2020) and this includes measurement of cPLI (Cridge, Mackin et al. 2020). It has been proven over the decade that the PLI assay development, analytical validation, and evaluation is very useful for the diagnosis of pancreatitis in both dogs and cats (Xenoulis and Steiner 2012)( Xenoulis PG, Steiner JM, 2013). Pancreatic lipase is expressed exclusively by pancreatic acinar cells and thus plays an important role in assessing the exocrine pancreas (Steiner, Berridge et al. 2002, Steiner, Rutz et al. 2006, Neilson-Carley, Robertson et al. 2011, Xenoulis and Steiner 2012). The serum cPLI has been reported to detect specifically localized lipase in pancreatic acinar cells (Steiner, Berridge et al. 2002, Dröes and Tappin 2017). This makes increase in serum PLI concentrations unlikely coming from the lipase of other tissues (Lidbury and Suchodolski 2016). Considering the localization of the pancreatic lipase and the capacity of immunoassays to detect the unique protein structure of pancreatic lipase are known to be the advantages of measuring the serum cPLI during the diagnosis of exocrine pancreas diseases, resulting to high analytic specificity (Steiner,Rutz et al. 2006, Xenoulis and Steiner 2012) (Steiner JM, 2000; Hoffmann WE, 2008) (Steiner, Berridge et al. 2002, Neilson-Carley, Robertson et al. 2011). Nowadays, diagnosis of canine pancreatitis has mostly considered serum cPLI assays as the most specific serum biomarkers (Neilson-Carley, Robertson et al. 2011, Trivedi, Marks et al. 2011, Mawby, Whittemore et al. 2014). However, the serum cPLI concentration doesn’t define the severity of pancreatitis (Steiner, Newman et al. 2008, Trivedi, Marks et al. 2011, Xenoulis and Steiner 2012). References: Hoffmann WE. Diagnostic enzymology of domestic animals. In: Kaneko JJ, Harvey JW, Bruss ML, eds. Clinical Biochemistry of Domestic Animals. 6th ed. Burlington, MA: Academic
