Pneumonia and gastritis in a cat caused by feline herpesvirus-1
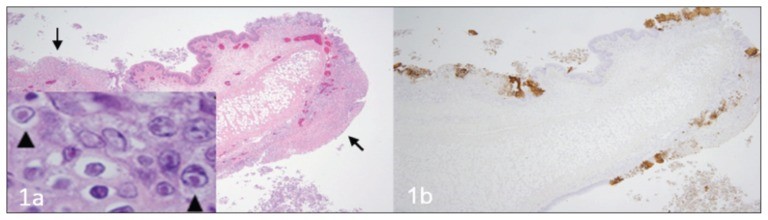
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4712990/ A fatal respiratory and gastric herpesvirus infection in a vaccinated, 6-year-old neutered male domestic shorthair cat with no known immunosuppression or debilitation. Histology examination revealed severe necrotizing bronchopneumonia, fibrinonecrotic laryngotracheitis, and multifocal necrotizing gastritis associated with eosinophilic intranuclear inclusion bodies in affected tissues of larynx, trachea, lung and stomach. Immunohistochemistry also displayed strong immunoreactivity for FHV-1 in the corresponding section of larynx, trachea, lung and stomach. Figure 1 Larynx. Cat. a — Note the multifocal areas of ulceration (arrows) and inflammation. Inset: inclusion bodies (arrowheads) within epithelial cells adjacent to areas of ulceration. H&E. b — Immunohistochemistry of the corresponding section of larynx displaying strong multifocal immunoreactivity for FHV-1. Figure 2 Trachea. Cat. a — Note the denuded tracheal mucosa covered by a thick fibrinonecrotic exudate (asterisks), attenuation of the epithelium lining the tracheal glands (arrows) and numerous inflammatory cells infiltrating the tracheal wall. Inset: Inclusion bodies within the epithelial cells lining the tracheal glands (arrowheads). H&E. b — Strong immunoreactivity for FHV-1 in the epithelium of the trachea and tracheal glands. Figure 3 Lung. Cat. a — Severe neutrophilic necrotizing bronchopneumonia affecting airways (asterisks) and surrounding tissues. H&E. b — Strong immunoreactivity for FHV-1 in bronchial and bronchiolar epithelium. Figure 4 Stomach. Cat. a — Area of necrosis within the gastric mucosa (asterisk). H&E. b — Multifocal areas of FHV-1 immunoreactivity corresponding to areas of necrosis within the gastric mucosa.
Feline Tritrichomonas Foetus Infection: A Review
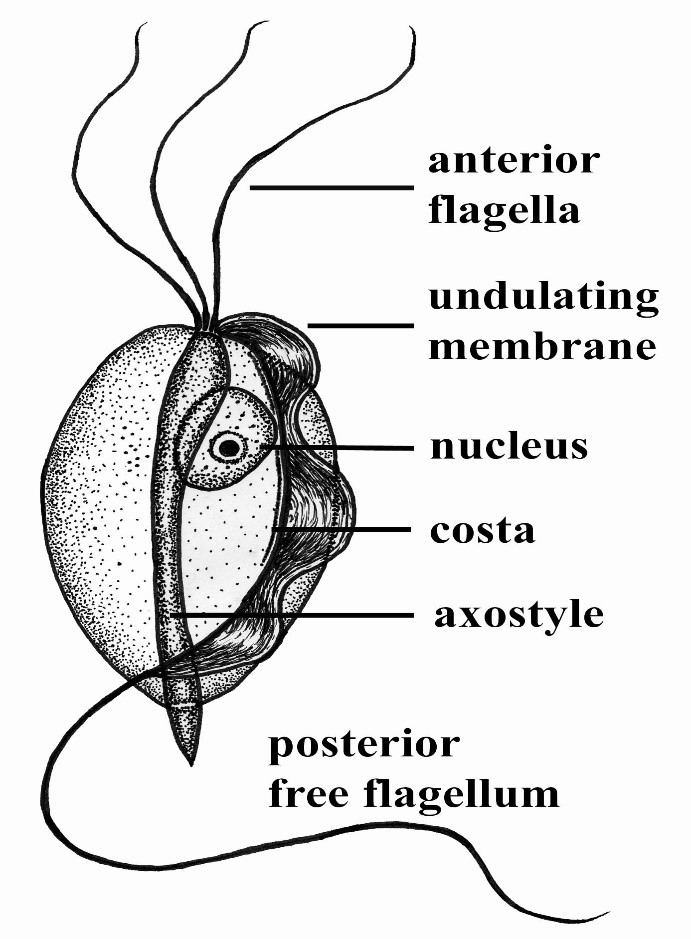
Maigan Espinili Maruquin Structure and Epidemiology The protozoan parasite Tritrichomonas foetus has sudden emergence of its syndrome in the 1990s causing feline intestinal tritrichomoniasis and has then attracted feline medicine studies (Levy, Gookin et al. 2003, Bell, Gowan et al. 2010) (Levy, Gookin et al. 2001 ). At a range of 10- 31%, infected cats in UK and USA comes from young, pedigree cats in multi-cat environment (Gookin, Levy et al. 2001, Foster, Gookin et al. 2004, Gookin, Stebbins et al . 2004, Gunn-Moore, McCann et al. 2007, Frey, Schild et al. 2009, Stockdale, Givens et al. 2009)( Gunn-Moore D, Tennant B, 2007)(Bell, Gowan et al. 2010). An estimate 30% of purebred cats in USA are suggested infected by T. foetus (Gookin, Stebbins et al. 2004, Gookin, Stauffer et al. 2010). The T. foetus , like other trichomonads, has only trophozoite stage, however, it has a pseudocyst stage (Lipman NS, et. al., 1999) (Pereira-Neves, Ribeiro et al. 2003, Benchimol 2004, Mariante, Lopes et al. 2004, Yao and Köster 2015). Therefore, cats are presumed to be infected via direct contact due to the absence of the cyst stage (Zajac AM, Conboy GA, 2006). The parasite is pear- or spindle-shaped, having three anterior flagella and one posterior flagellum. With its size of approximately 10-25 μm in length and 3-15 μm in width, the undulating membrane extends along the whole length of the body which emerges as the posterior flagellum. This trophozoite reproduces asexually by longitudinal binary fission (Yao and Köster 2015). (https://www.k-state.edu/parasitology/625tutorials/Protozoa10.html) Fig. 01. Morphologic structure of the Tritrichomonas foetus Infected cats were reported to experience chronic large bowel diarrhea as the parasite was found in the feline intestine (Foster, Gookin et al. 2004, Payne and Artzer 2009). Reports also showed the presence of the organism in the feline uterus (Dahlgren, Gjerde et al. 2007). Further, the isolates of T. foetus from cattle are known to be infectious for the cats, while the isolates of the same species from cats are infectious to cattle (Stockdale, Dillon et al. 2008, Walden, Rodning et al. 2008, Payne and Artzer 2009). Clinical Signs/ Pathogenesis Despite suggestions of strong association of feline T. foetus and chronic diarrhea (Gookin, Stebbins et al. 2004, Mardell and Sparkes 2006, Gunn-Moore, McCann et al. 2007, Burgener, Frey et al. 2009, Holliday, Deni et al. 2009, Pham 2009, Stockdale, Givens et al. 2009, Kuehner, Marks et al. 2011), whether the parasite alone is sufficient to cause clinical signs or the foetus-associated diarrhea, being a primarily multifactorial disease involves concurrent infection with other enteropathogens, host and environmental factors (Gookin, JL, et. al., 1999) (Gookin, Levy et al. 2001, Bissett, Gowan et al. 2008, Stockdale, Givens et al. 2009, Kuehner, Marks et al. 2011). It is possible that the trophozoites are transmitted by a fecal- oral route from an infected to uninfected cat (Yao and Köster 2015). Symptoms may appear early as 2 to 7 days after orogastric inoculation (Gookin, Levy et al. 2001, Yao and Köster 2015). Whereas, infected cats showed anorexia, depression, vomiting and weight loss while experimental infections also reported vomiting and fever (Mardell and Sparkes 2006, Xenoulis, Lopinski et al. 2013, Yao and Köster 2015)( Stockdale, H., et al., 2007). Chronic large bowel diarrhea, associated with blood, mucus, flatulence, tenesmus, and anal irritation were also reported (Foster, Gookin et al. 2004, Gookin, Stebbins et al. 2004, Payne and Artzer 2009, Stockdale, Givens et al. 2009). With the characteristic of the trichomonads as commensal organisms, some hosts show no clinical signs and are asymptomatic (Payne and Artzer 2009). In some studies, the parasite, with its surface located antigen, was detected on epithelial surface and within the superficial detritus of the cecal and colonic mucosa (Gookin, Levy et al. 2001, Yao and Köster 2015), while naturally infected cats showed parasite in close proximity to the mucosal surface and less frequently in the lumen of colonic crypts (Yaeger and Gookin 2005, Yao and Köster 2015). Conclusively, T. foetus trophozoites can be detected in epithelial surface and crypts of cecum and colon (Yao and Köster 2015). Mechanisms were then described to include possibilities of alterations in the normal intestinal flora, adherence to the epithelium, and elaboration of cytokines and enzymes (Payne and Artzer 2009). Diagnosis For cats <6months old with recent clinical signs of chronic large bowel diarrhea, infection with T. foetus infection is suspected (Yao and Köster 2015). Diagnosis of the T. foetus infection may be conducted via direct observation of the flagellates in fresh or cultured feces (Payne and Artzer 2009) or on a saline diluted direct fecal smear (Yao and Köster 2015). The trophozoites of T. foetus are difficult to distinguish from Giardia spp and other nonpathogenic intestinal trichomonads. However, although the size of T. foetus and Giardia spp are almost the same, they move differently. The movement of Giardia spp. resembles the fall of a leaf while trichomonads move erratically (Yao and Köster 2015)( Gookin JL, Levy MG, 2008). Feline feces can be cultivated and be tested in commercially available InPouch™ TF medium (Payne and Artzer 2009, Yao and Köster 2015) or DNA extraction and amplification of T. foetus rDNA by the use of PCR from feces samples can be conducted (Gookin, Stebbins et al. 2004, Manning 2010, Yao and Köster 2015). Moreover, other causes of diarrhea, like bacterial, viral, other parasites, and nutritional problems should be ruled out before a diagnosis of tritrichomoniasis can be made (Payne and Artzer 2009). Treatment and Disease Managements The T. foetus infection in cats has no approved treatment. While treating infected animals is difficult, success is also limited (Payne and Artzer 2009). Literatures have used therapeutics including paromomycin, fenbendazole, furazolidone, nitazoxanide, metronidazole, tinidazole and ronidazole (Gookin, Breitschwerdt et al. 1999, Gookin, Levy et al. 2001, Yao and Köster 2015). The ronidazole is not registered for human or veterinary use (Yao and Köster 2015) however, it showed effectiveness in experimentally infected cats (Gookin, Copple et al. 2006) (Gookin JL, Dybas D, 2008). Due to possible neurologic side effects, caution is observed
Leptospirosis
Andy Hua Introduction Classification Taxonomy Classically, the genus Leptospira was divided into 2 species based on genetic analysis: L. interrogans sensu latu (pathogenic strains) and L. biflexa sensu latu (saprophytic strains). L. interrogans is divided into more than 250 serovars based on antigenic composition and further classified into antigenically related serogroups. There is a serovar spectrum and frequency that differs according to countries and regions (depending on distribution of rodent hosts, import of dogs from abroad, use of vaccination). The main infecting serovars in dogs were Icterohaemorrhagiae and Canicola in Europe and America prior to 1960. Since the use of the bivalent vaccine against Canicola and icterohaemorrhagiae , OTHER serovars A to the Shift occurred .Besides. L. icterohaemorrhagiae and L. canicola , serovars Importance of Dogs in the include: grippotyphosa , Bratislava , Saxkoebing , Sejroe , Copenhagi , Australis , Bataviae , and Pomona , autumnalis , and hardjo . Icterohaemorrhagiae and Canicola infections in unvaccinated dogs still occur, indicating that these serovars are not fully eradicated. Leptospires are motile, obligate aerobe, gram-negative bacteria, which are not visible in routinely fixed smears. Dark field microscopy (Fig. 1) or phase contrast microscopy is necessary for visibility of unstained leptospires. Clinical Effects Epidemiology Habitat Leptospires have been isolated from birds, reptiles, amphibians and invertebrates. Rodents and wild carnivores are the most frequent carriers. Reservoir hosts show few or no signs of disease. Leptospira spp. are commonly sequestered in the renal tubules of mammalian kidneys. Different serovars typically have different reservoir hosts. Lifecycle Generation time in culture media or host is long. Transmission Direct or indirect transmissions are possible. Indirect transmission through contaminated water or soil is more common. Pathological effects Infection occurs through ingestion of infected rodents or penetration of mucosae or traumatized skin. Leptospiremia occurs within 1 week. Leptospires spread to other organ systems (kidneys, liver, spleen, endothelial cells, lungs, uvea/retina, skeletal and heart muscles, pancreas, and genital tract) and cause tissue damage, visceral and vascular inflammation. Leptospiral pulmonary hemorrhage syndrome (LPHS) Lung: pulmonary hemorrhage can occur as severe manifestation of acute leptospirosis. Leptospires can persist in immune privileged site (eg, renal tubes, eye). In the presence of adequate antibody titers, leptospires are eliminated from most organs. In the presence of low antibody titers mild leptospiremia can continue with a subclinical course of disease. Other Host Effects Individual host may show little or no clinical signs but may be source of infection in the same animal species. An animal that has recovered may become a long-term shedder of the organism. Mainly dogs show disease, rodents often the reservoir. Cat disease is uncommon, but serology shows that asymptomatic infection occurs. Individual host can show little or no clinical signs but can be source of infection to other animals or humans. An animal that has recovered can become a long-term shedder of the organism. Rodents are often the reservoir. Dogs commonly succumb to disease if infected. In cats, disease is uncommon but asymptomatic infection and shedding in urine occurs. Control Control via animal Antimicrobial therapy Dogs with gastrointestinal signs should initially be treated with intravenous penicillin derivates (eg, ampicillin or amoxicillin 20-30 mg/kg q6-8h). These should be continued until gastrointestinal signs are under control and liver enzymes are normalized. A directly following antimicrobial therapy with 3 weeks of oral doxycycline (5 mg/kg q12h) is necessary for prevention of carrier states. Dogs without gastrointestinal signs should immediately be treated with doxycycline. Antibody testing of dogs living in the same household as infected dogs is recommended. Oral doxycycline (5 mg/kg q12h for 3 weeks) should be administered, if these dogs have antibodies. Symptomatic treatment Treatment of dogs with gastrointestinal sings includes antiemetics, gastroprotectants, and nutritional support. Use of opioids in dogs with pain can be necessary. Treatment of dogs with acute kidney injury (AKI) includes correction of loss of fluid, electrolytes, acid-base imbalances and hypertension, and if necessary hemodialysis for patients with persistent oligoanuria, life-threatening hyperkalemia, or severe volume overload. Oxygen therapy or mechanical ventilation can be necessary in dogs with LPHS. Plasma transfusions can be necessary for patients with DIC (disseminated intravascular coagulation). Whole blood transfusion can be helpful, if bleeding occurs. Hemodialysis Hemodialysis is necessary in dogs with acute renal failure (life-threatening hyperkalemia or severe volume overload) and in dogs with advanced uremia refractory to medical management. Early referral to facilities where hemodialysis is available is recommended. Renal recovery usually occurs after 2-7 days of dialytic support. Hemodialysis leads to favourable prognosis for renal recovery (in more than 80% of dogs). Mechanical ventilation Anesthesia ventilators: overview can be necessary in dogs with severe pulmonary hemorrhage due to LPHS. Vaccination Vaccination protects against clinical disease and carrier status with shedding. Protection is serogroup-specific and temporary. Annual boosters are required. Diagnosis Leptospirosis at its onset is often misdiagnosed as aseptic meningitis, influenza, hepatic disease or fever (pyrexia) of unknown origin. Despite being common, the diagnosis of leptospirosis is often not made unless a patient presents with textbook manifestations of the so called Weil’s disease, such as fever plus jaundice, renal failure and pulmonary haemorrhage. Leptospiral infection often has minimal or no clinical manifestations; of the cases in which fever develops, as many as 90% are undifferentiated febrile illnesses. Moreover, clinicians may fail to recognize that transmission of leptospirosis can occur in the urban setting because it is incorrectly perceived to be a rural disease. Therefore, diagnosis is based on laboratory tests rather than on clinical symptoms alone. In developing countries,Laboratory facilities may be inadequate for diagnosis a high prevalence of the disease. Of substantial clinical importance despite the syndrome of leptospiral pulmonary haemorrhage has emerged in recent years, in diverse places around the world. Two important issues continue to confront clinicians regarding leptospirosis. The first is how to reliably establish the diagnosis. The most common way to diagnose leptospirosis is through serological tests either the Microscopic Agglutination Test (MAT) which detects serovar-specific antibodies, or a solid- phase assay for the detection of Immunoglobulin M (IgM) antibodies. Leptospira are present in the blood until they are
Bartonella henselae: An Infectious Pathogen among Cats
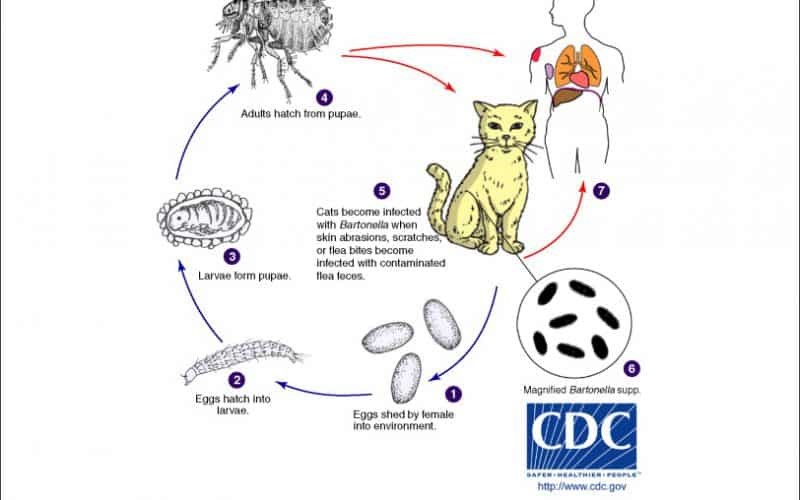
Table of Contents Maigan Espinili Maruquin 1. Introduction Overview of Bartonella henselae as a Significant Feline and Zoonotic Pathogen Bartonella henselae is a small, fastidious, Gram-negative, facultative intracellular bacterium with a global distribution. It exhibits a marked tropism for endothelial cells and erythrocytes, enabling the establishment of chronic, relapsing bacteremia that may persist for months or even years in infected hosts. Domestic cats are the primary mammalian reservoir and represent the principal source of zoonotic transmission to humans. Kittens and feral cats typically harbor higher bacterial loads, although subclinical infection is widespread across the global feline population. Reported bacteremia prevalence in apparently healthy cats ranges from 8% to 56%, depending on geographic region and flea exposure. The primary competent vector for B. henselae is the cat flea (Ctenocephalides felis), within which the organism replicates in the flea gut and is excreted in flea feces (commonly referred to as flea dirt). These contaminated feces can remain infectious in the environment for at least nine days, facilitating indirect transmission. Importance in Companion Animal Medicine and Public Health From a companion animal medicine perspective, B. henselae is clinically significant because most infected cats function as asymptomatic carriers, silently sustaining zoonotic risk. Nevertheless, increasing evidence links B. henselae infection to sporadic but severe feline disease manifestations, including endocarditis, myocarditis, and ocular inflammatory conditions such as uveitis. In dogs, which are considered accidental hosts, bartonellosis is often more pathogenic and has been strongly associated with culture-negative endocarditis and granulomatous inflammatory disease. In public health, B. henselae is best known as the primary etiological agent of Cat Scratch Disease (CSD) in humans. Transmission most commonly occurs when cat claws or oral cavities become contaminated with infected flea feces, which are then inoculated into human skin through a scratch or bite. Immunocompetent individuals typically develop a self-limiting illness characterized by regional lymphadenopathy, fever, and a papule at the site of inoculation. Immunocompromised individuals, including those with HIV/AIDS or organ transplant recipients, are at risk for severe and potentially fatal complications, such as bacillary angiomatosis, bacillary peliosis, encephalitis, and endocarditis, reflecting the organism’s vasoproliferative potential. Scope of the Review and Relevance to Clinical Practice A clear understanding of the epidemiology, pathogenesis, and persistence mechanisms of B. henselae is essential for effective clinical management and disease prevention. Diagnosis remains particularly challenging, as the organism is highly fastidious and slow-growing, frequently resulting in false-negative blood culture findings and so-called “culture-negative” infections. Although molecular assays such as PCR and serological testing are widely employed, interpretation is complicated by intermittent bacteremia and the high background seroprevalence among healthy cats. In clinical practice, adoption of a One Health framework is critical. Veterinarians play a central role in mitigating zoonotic risk through owner education, emphasizing strict, year-round flea control, appropriate hygiene, and cautious interaction with cats, especially in households containing immunocompromised individuals. Management is further complicated by the absence of a standardized antimicrobial protocol capable of reliably achieving complete bacteriological clearance in feline hosts. To conceptualize its biological behavior, B. henselae may be likened to a “stealthy hitchhiker.” By residing within erythrocytes and vascular endothelium, the organism evades immune surveillance, periodically re-emerging only to secure transmission via a passing flea. 2. Characteristics and Epidemiology 2.1 Taxonomy and Microbiological Characteristics The genus Bartonella comprises small, thin, fastidious, and pleomorphic Gram-negative bacilli. These organisms are facultative intracellular pathogens with a highly specialized biological niche characterized by a pronounced tropism for endothelial cells and erythrocytes (red blood cells). Following host entry, Bartonella spp. proliferate within membrane-bound vacuoles, often referred to as invasomes, inside vascular endothelial cells. Periodic release into the bloodstream allows subsequent invasion of erythrocytes, within which the bacteria may persist until cellular senescence or destruction occurs (Cunningham and Koehler 2000; LeBoit 1997). Transmission is predominantly arthropod-borne, involving vectors such as fleas (Ctenocephalides felis), ticks, lice, and sand flies. Although Bartonella species have been isolated from a wide range of mammalian hosts, including rodents, rabbits, canids, and ruminants, domestic and feral cats represent the principal mammalian reservoir for the most epidemiologically and clinically significant zoonotic species, particularly B. henselae (Chomel et al., 1996; Pennisi, Marsilio et al., 2013; Guptill, 2012). 2.2 Global Distribution and Seroprevalence Bartonella species exhibit a global distribution, although prevalence varies markedly according to environmental and ecological conditions. In European feline populations, reported antibody prevalence ranges from 8% to 53% (Pennisi, Marsilio et al., 2013; Zangwill, 2013), while global serological evidence of exposure in cats spans approximately 5% to 80% (Guptill, 2012). The epidemiology of Bartonella infection is strongly influenced by geography, climate, and flea density. The highest prevalence rates are consistently observed in warm, humid temperate and tropical regions, where environmental conditions favor the survival and propagation of C. felis. In contrast, in colder climates, such as Norway, Bartonella infection in cats is reported to be rare or virtually absent, reflecting the limited persistence of flea vectors under such conditions. 2.3 Species Diversity and Genotypes Although 22 to 38 Bartonella species have been described to date, Bartonella henselae remains the most frequently detected species in both domestic cats and humans. Feline populations may also harbor Bartonella clarridgeiae, identified in approximately 10% of infected cats, while B. koehlerae is detected far less commonly (Guptill, 2012). Considerable regional variation exists among B. henselae genotypes, which are broadly classified into Houston-1 (Type I) and Marseille (Type II) strains. Type II (Marseille) predominates among feline populations in the western United States, western continental Europe, the United Kingdom, and Australia. Type I (Houston-1) is the dominant genotype in Asia, including Japan and the Philippines, and is most frequently isolated from human clinical cases worldwide, even in regions where Type II strains are more prevalent among cats. Beyond domestic cats, Bartonella infections have been documented in non-domestic felids, including African lions, cheetahs, and various neotropical wild cat species, underscoring the broad ecological adaptability of the genus (Guptill, 2012). To conceptualize its ecological behavior, Bartonella may be viewed as a “weather-dependent squatter.” It establishes itself most successfully in warm, densely populated environments rich in flea vectors,
Feline Pancreatic Lipase (fPL)
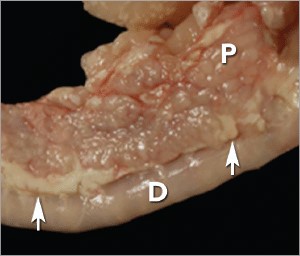
Andy Pachikerl, Ph.D Introduction Pancreatitis appears to be a common disease in cats,1 yet it remains frustratingly difficult to establish a clinical diagnosis with certainty. Clinicians must rely on a combination of compatible clinical findings, serum feline pancreatic lipase (fPL) measurement, and ultrasonographic changes in the pancreas to make an antemortem diagnosis, yet each of these 3 components has limitations. Acute Versus Chronic Pancreatitis Acute pancreatitis is characterized by neutrophilic inflammation, with variable amounts of pancreatic acinar cell and peripancreatic fat necrosis (Figure 1).1 Evidence is mounting that chronic pancreatitis is more common than the acute form, but sonographic and other clinical findings overlap considerably between the 2 forms of disease.1-3 Diagnostic Challenges Use of histopathology as the gold standard for diagnosis has recently been questioned because of the potential for histologic ambiguity.3,4 A seminal paper exploring the prevalence and distribution of feline pancreatic pathologic abnormalities reported that 45% of cats that were apparently healthy at time of death had histologic evidence of pancreatitis.1 The 41 cats in this group included cats with no history of disease that died of trauma, and cats from clinical studies that did not undergo any treatment (control animals). Conversely, multifocal distribution of inflammatory lesions was common in this study, raising the concern that lesions could be missed on biopsy or even necropsy. Prevalence Such considerations help explain the wide range in the reported prevalence of feline pancreatitis, from 0.6% to 67%.3 The prevalence of clinically relevant pancreatitis undoubtedly lies somewhere in between, with acute and chronic pancreatitis suggested to represent opposite points on a disease continuum.2 FIGURE 1. Duodenum (D) and duodenal limb of the pancreas (P) in a cat with acute pancreatitis and necrosis; well-demarcated areas of necrosis are present at the periphery of the pancreas in the peripancreatic adipose tissue(arrows). Courtesy Dr. Arno Wuenschmann, Minnesota Veterinary Diagnostic Laboratory Risk factors No age, sex, or breed predisposition has been recognized in cats with acute pancreatitis, and no relationship has been established with body condition score.3-5 Cats over a wide age range, from kittens to geriatric cats, are affected; cats older than 7 years predominate. In most cases, an underlying cause or instigating event cannot be determined, leading to classification as idiopathic.3 Abdominal trauma, sometimes from high-rise syndrome, is an uncommon cause that is readily identified from the history.6 The pancreas is sensitive to hypotension and ischemia; every effort must be taken to avoid hypotensive episodes under anesthesia. Comorbidities In cats with acute pancreatitis, the frequency of concurrent diseases is as high as 83% (Table 1).2 Pancreatitis complicates the management of some diabetic cats and may induce, for example, diabetic ketoacidosis.7 Anorexia attributable to pancreatitis can be the precipitating cause of hepatic lipidosis.8 The role of intercurrent inflammation in the biliary tract or intestine (also called triaditis) in the pathogenesis of pancreatitis is still uncertain. Roles of Bacteria In one study, culture-independent methods to identify bacteria in sections of the pancreas from cats with pancreatitis detected bacteria in 35% of cases.9 This report renewed speculation about the role of bacteria in the pathogenesis of acute pancreatitis, and the potential role that the common insertion of the pancreatic duct and common bile duct into the duodenal papilla may play in facilitating reflux of enteric bacteria into the “common channel” in cats. Awareness of triaditis may affect the diagnostic evaluation of individual patients. Table 1. Clinical Data from 95 Cats with Acute Pancreatitis (1976—1998; 59% Mortality Rate) & 89 Cats Diagnosed with Acute Pancreatitis (2004—2011; 16% Mortality Rate) PARAMETER HISTORICAL DATA* CATS WITH PANCREATITIS† SURVIVING CATS WITH PANCREATITIS† Number of Cats 95 89 75 ALP elevation 50% 23% 18% ALT elevation 68% 41% 36% Apparent abdominal pain 25% 30% 32% Cholangitis NA 12% 11% Concurrent disease diagnosed NA 69% 68% Dehydration 92% 37% 42% Diabetic ketoacidosis NA 8% 5% Diabetes mellitus NA 11% 12% Fever 7%‡ 26% 11% GGT elevation NA 21% 18% Hepatic lipidosis NA 20% 19% Hyperbilirubinemia 64% 45% 53% Icterus 64% 6% 6% Vomiting 35%—52% 35% 36% ALP = alkaline phosphatase; ALT = alanine aminotransferase; GGT = gamma glutamyl transferase; NA = not available * Summarized from 4 published case series; a total of 56 cats had acute pancreatitis diagnosed at necropsy and 3 by pancreatic biopsy5,8,10,11 † Data obtained from reference12 ‡ 68% of cats were hypothermic DIAGNOSTIC EVALUATION Many cats with pancreatitis have vague, nonspecific clinical signs, which make diagnosis challenging.5 Clinical signs related to common comorbidities, such as anorexia, lethargy, and vomiting, may overlap with, or initially mask, the signs associated with pancreatic disease. Early publications on the clinical characteristics of acute pancreatitis required necropsy as an inclusion criterion, presumably skewing the spectrum of severity of the reported cases.5,8,10,11 Cats with chronic pancreatitis were excluded from these reports. Clinical Findings Table 1 lists common clinical findings in cats from necropsy-based reports and a recent series of 89 cats with acute pancreatitis studied by the authors.12 Note the lower prevalence of most clinical findings in the cats diagnosed clinically rather than from necropsy records. In our evaluation of affected cats, 17% exhibited no signs aside from lethargy and 62% were anorexic. Vomiting occurs inconsistently (35%—52% of cats). Abdominal pain is detected in a minority of cases even when the index of suspicion of pancreatitis is high. About ¼ of cats with pancreatitis have a palpable abdominal mass that may be misdiagnosed as a lesion of another intra-abdominal structure. Laboratory Analyses Hematologic abnormalities in cats with acute pancreatitis are nonspecific; findings may include nonregenerative anemia, hemoconcentration, leukocytosis, or leukopenia. Serum biochemical profile results vary (Table 1). In our acute pancreatitis case series, 33% of cats had no abnormalities in their chemistry results at presentation.12 Serum cholesterol concentrations may be high in up to 72% of cases. Some cases of acute pancreatitis are associated with severe clinical syndromes, such as shock, disseminated intravascular coagulation, and multiorgan failure, that influence some serum parameters, such as albumin, liver enzymes, and coagulation tests. Plasma ionized calcium concentration may be low, and has
Concurrent with T-zone lymphoma and high-grade gastrointestinal cytotoxic T-cell lymphoma in a dog
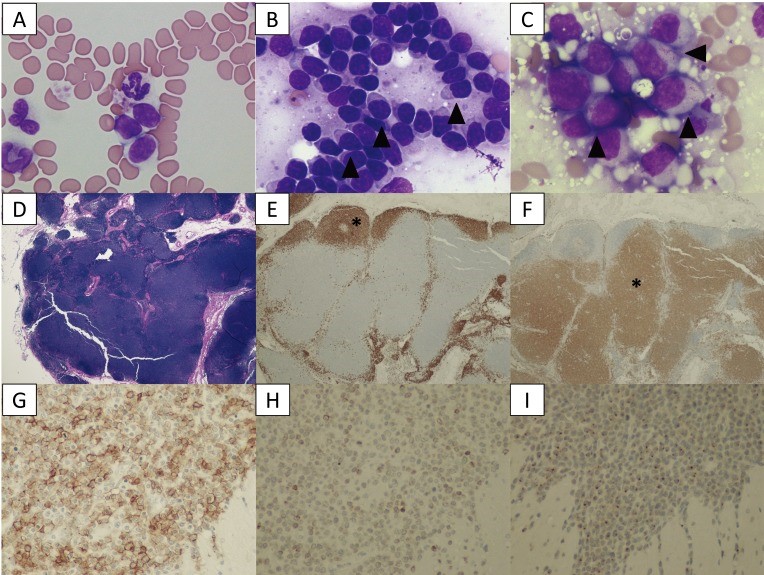
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5402196/ A 9-year-old, spayed female Golden Retriever dog showed lymphocytosis and lymphadenopathy, secondary to suspected chronic lymphocytic leukemia (CLL). Small-to-intermediate lymphocytes were observed from the cytological examination of the right popliteal lymph node via a fine-needle aspirate. The dog was suspected to have a low-grade lymphoma based on the finding of cytology. Also, ultrasonography reveled thickened lesions in the stomach and small intestine. Histopathology of the popliteal lymph node and small intestine revealed a simultaneous presence of T-zone lymphoma (TZL) and high-grade gastrointestinal (GI) cytotoxic T-cell lymphoma. PCR for antigen receptor rearrangements assay suggested that both lymphomas, though both originated in the T-cells, derived from different genes. The dog died 15 days after diagnosis, despite chemotherapy. Fig. 1. A–C: Cytological images on day 1. (A) Peripheral blood smear. Increased numbers of small lymphocytes. (B) Cytology of the popliteal lymph node biopsy. Most lymphocytes are small-to-intermediate, mature lymphocytes. Some lymphocytes show a “hand mirror” type of cytoplasmic extension (arrowhead) (Wright-Giemsa stain, × 400). (C) Slide preparation of tissue from the small intestine. The lymphocytes are intermediate-to-large, immature cells, and some display azurophilic granules in the cytoplasm (LGLs, arrowhead). D–F: Histological images of popliteal lymph node tissue. (D) Hematoxylin and eosin (H&E) staining. (E) The lymphocytes with fading follicular structures are CD20 positive (asterisk). Immunolabeling with anti-CD20, a hematoxylin counterstain. (F) The nodal capsule (CD3 positive) is thinned without the involvement of the perinodal tissue (asterisk). Immunolabeling with anti-CD3, hematoxylin counterstain. G–I: Histological images of the intestinal tissue. All lymphocytes are positive for CD20 (G), CD3 (H) and granzyme B (I). Fig. 2. (A) Transverse ultrasound image on day 1 showing a thickened intestinal wall (approximately 9.0 mm, arrowhead). (B) Post-contrast transverse CT image on day 2 also showing a thickened intestinal wall (arrowheads). The intrathoracic and abdominal lymph nodes are enlarged. Fig. 3. PARR analysis. (A) The peripheral blood sample shows TCRγ gene rearrangement. (B) The intestinal tissue sample also shows TCRγ gene rearrangement. The two tumors demonstrate clonal expansions from different primers.
Case study: Primary cardiac lymphoma in a 10-week-old dog
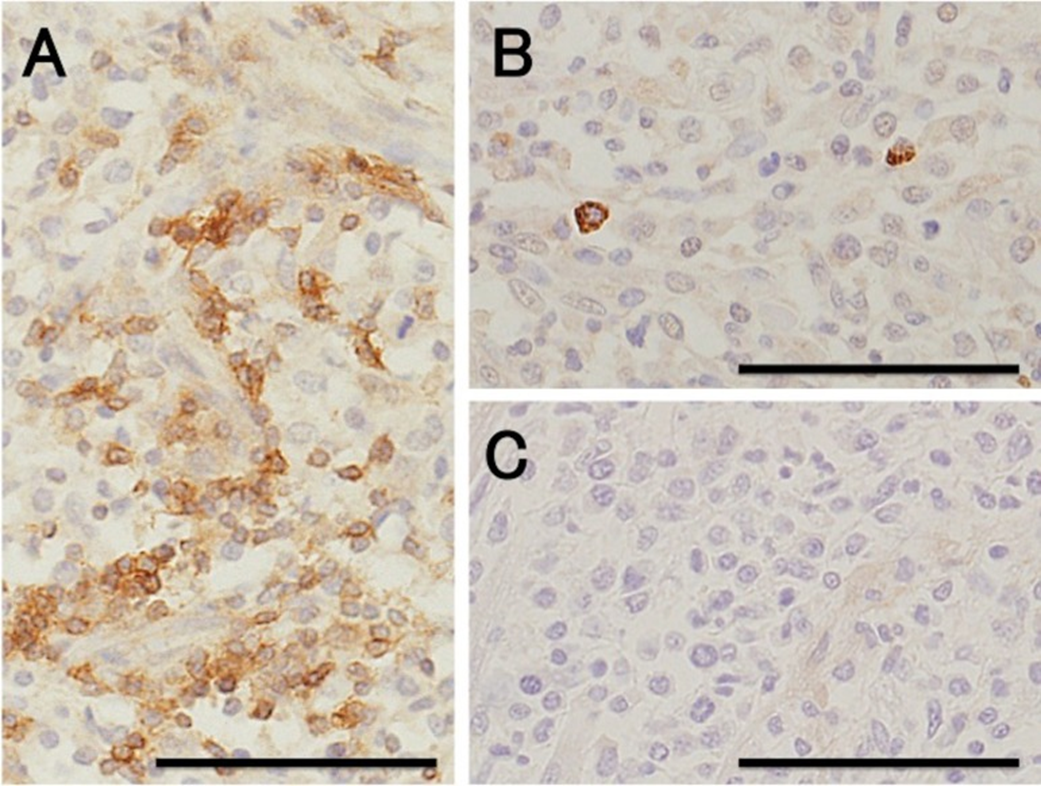
Case study: Primary cardiac lymphoma in a 10-week-old dog Robert Lo, Ph.D, D.V.M Original: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6261812/ Canine lymphoma usually appears in multicentric, alimentary, mediastinal, and cutaneous forms, but rarely affects only heart. This case reports a uncommon primary cardiac lymphoma (PCL) of a 10-week-old miniature dachshund. The dog clinically showed acute onset of weakness. Electrocardiography indicated sustained ventricular tachycardia, and thoracic and abdominal radiography revealed pleural and peritoneal effusion. Echocardiography revealed severely hypokinetic left and right ventricles. After failure of treatment, the dog died about 1 hr after admission and underwent autopsy. Gross examination of a longitudinal section through the entire heart revealed poorly demarcated focal or patchy areas of grayish-white tissue infiltrating extensively into the myocardium. Histologically, these lesions were consistent with infiltrative proliferation of neoplastic lymphoid cells. Immunohistochemical staining confirmed the diagnosis of PCL of T-cell origin. There have been no previous reports of such young dogs with PCL. Fig. 1. Six lead electrocardiographic tracings from the 10-week-old dog, showing monomorphic ventricular tachycardia, rate 360 beats per minute, almost regular (bipolar standard limb leads; 50 mm/sec). Fig. 2. Formalin-fixed heart transected along the long axis, showing extensive infiltration of grayish-white neoplastic tissue into the myocardium of the entire heart. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. Scale: 1 mm. Fig. 3. (A) Microscopic section taken from the ventricular septum, showing marked infiltrative proliferation of neoplastic lymphoid cells in the myocardium. Sheets of neoplastic round cells separate individual muscle fibers. HE. Bar: 50 µm. (B) The outlined square area in A is shown at higher magnification. HE. Bar: 20 µm. Fig. 4. Immunohistochemical labeling of the neoplastic lymphoid cells. Hematoxylin counterstain. Bar: 50 µm. (A) A large number of neoplastic cells stain positively for CD3. (B) Fewer neoplastic cells stain positively for CD79α. (C) All the neoplastic cells are negative for CD20.
What are Feline Injection-Site Sarcomas (FISS)?
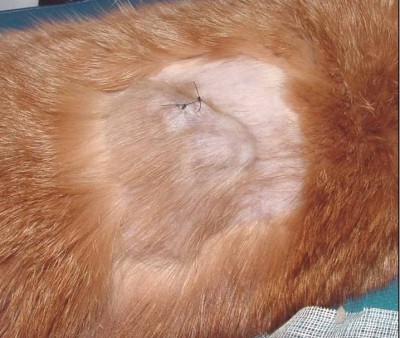
What are Feline Injection-Site Sarcomas (FISS)? Maigan Espinili Maruquin I. Characteristics / Epidemiology The feline injection-site sarcomas (FISS) were first reported on 1991 (Hendrick and Goldschmidt 1991). With the implementation of stricter vaccination and development of vaccines for rabies and FeLV, the increased incidence of vaccine reactions was recognized (Hendrick and Dunagan 1991, Kass, Barnes et al. 1993, Hartmann, Day et al. 2015, Saba 2017). With this, recommendations were to use the term ‘vaccine-associated sarcomas’, however, studies show that aside from vaccines are other non-vaccinal injectables in the subcutis or muscle can also cause chronic inflammatory response which led to reclassification as ‘feline injection-site sarcomas’ (FiSSs) (Martano, Morello et al. 2011, Hartmann, Day et al. 2015). The FISS develops in 1–10 of every 10,000 vaccinated cats wherein malignant skin tumors of mesenchymal origin develops (Zabielska-Koczywąs, Wojtalewicz et al. 2017). It has been described as secondary to inflammation in different organs like eye (PEIFFER, MONTICELLO et al. 1988), uterus (Jelínek 2003) and muscle or skin after placement of non-absorbable suture or microchips (Buracco, Martano et al. 2002) (Bowlt 2015). Between three months to 10 years after vaccination, the development of FISS can occur (Hendrick, Shofer et al. 1994, McEntee and Page 2001) (Esplin, D. G., et al., 1993). Whereas, a study reported that the younger cats developed tumor at the vaccination site as compared to the older ones with similar tumors in other body areas with bimodal distribution of age with a peak at 6–7 years and a second at 10–11 years (Kass, Barnes et al. 1993, Martano, Morello et al. 2011). Fig. 01. Saba, C. F. 2017 shows the occurrence of FISS. (https://doi.org/10.2147/VMRR.S116556) II. Pathogenesis / Clinical Signs After investigations, the hypothesis suggests that secondary to chronic and inflammatory response to vaccine or injection, having ultimate malignant transformation of surrounding fibroblasts and myofibroblasts triggers the tumors (Hendrick and Brooks 1994, Hartmann, Day et al. 2015, Saba 2017)( Hendrick MJ., 1999). Fig. 02. (Cecco, B.S., et al., 2019) The sites where FISS occurs (https://doi.org/10.1016/j.jcpa.2019.08.009) Reports show significant correlation between the rabies and/ or FeLV vaccinations in the development of FISS (Hendrick, Goldschmidt et al. 1992, Kass, Barnes et al. 1993, Hendrick, Shofer et al. 1994). Despite many causes are associated with what triggers the tumor, higher risks are seemed to be coming from vaccines, specifically adjuvanted (Hartmann, Day et al. 2015). Discovered were traces of adjuvants in the inflammatory reaction and later in histological sections (Hendrick and Brooks 1994, Hartmann, Day et al. 2015). Particles of grey- brown material in the necrotic centre and within the cytoplasm of macrophages were reported consistent with an inflammatory reaction (Hendrick and Dunagan 1991, Hendrick and Brooks 1994, Martano, Morello et al. 2011). The infiltrates reported includes macrophages often having cytoplasmic material, giant cells, lymphocytes and mixed neutrophils and eosinophils. Further, identified in the tumors were cytokines, growth factors and mutations in tumor suppressor genes (Ladlow 2013, Carneiro, de Queiroz et al. 2018). While fibrosarcoma is commonly diagnosed, some histological types were also reported to include: malignant fibrous histiocytoma, rhabdomyosarcoma, myxosarcoma, liposarcoma, nerve sheath tumor, poorly differentiated sarcomas, and extraskeletal osteosarcoma and chondrosarcoma (Esplin, McGill et al. 1993, Hendrick and Brooks 1994, Hershey, Sorenmo et al. 2000, Dillon, Mauldin et al. 2005, Saba 2017). According to Saba. C, 2017, any sarcoma that develops within the vicinity of vaccination or injection site should be considered an FISS and thus, should be treated aggressively. While tumors are invasive and variable in size, Martano M.E., et al, 2011 reported that large sized may be due to rapid growth. On the other hand, there could also be delayed in appearance due to its interscapular or deep location (Bowlt 2015). The mass can also be mobile or intensely adherent to the underlying tissue which is usually not painful, but solid and may be cystic (Bowlt 2015). These tumors that develop commonly in sites of injection can reach several centimetres in diameter within a few weeks (Martano, Morello et al. 2011). Since not all cats develop this tumor after vaccination, suggestions are due to genetic predisposition, with higher case of FISS occurrence in siblings of affected cats. Further, some cats may develop more than one FiSS (Hartmann, Day et al. 2015). III. Staging / Diagnosis To properly react with the tumor, proper staging shall be performed. Once a histological diagnosis has been confirmed (Bowlt 2015), it requires complete blood count, a serum biochemical panel, urinalysis, 3-view thoracic radiography, lymph node examination by palpation, and ultrasonography of the abdominal cavity and cytology when applicable (Séguin 2002, Zabielska-Koczywąs, Wojtalewicz et al. 2017). Abdominal ultrasound may be required, depending on the location of the tumor. Computed tomography (CT) or magnetic resonance imaging (MRI) of the lesion and the thorax is required to see the actual size and evaluate the extent of the tumor (Cronin, Page et al. 1998, McEntee and Page 2001, Martano, Morello et al. 2011, Rousset, Holmes et al. 2013, Travetti, di Giancamillo et al. 2013, Saba 2017, Zabielska-Koczywąs, Wojtalewicz et al. 2017). Thoracic radiography is then performed to exclude metastatic deseases, which has 10- 24% chances (Saba 2017, Zabielska-Koczywąs, Wojtalewicz et al. 2017). Whereas, there is as high as 45% for the recurrence rate even after performing surgical excision (Cronin, Page et al. 1998) IV. Treatment Considering the possibility of misdiagnosing the tumor as a granuloma from small tissue samples, and the fact that these can be heterogeneous, incisional biopsy can be done at sites that can be easily excised (Martano, Morello et al. 2011). The indications for a biopsy are based in 3-2-1 rule (Vaccine-Associated Feline Sarcoma Task Force, 2005; Vaccine-Associated Feline Sarcoma Task Force guidelines, 1999; (Morrison and Starr 2001). This incisional biopsy is strongly recommended for masses that has persisted for >3 months, is >2 cm, and/or is growing over the course of 1 month post injection in the site (Saba 2017). Radical surgery or wide excision may be recommended. Surgery will be
Canine Anaplasmosis

Canine Anaplasmosis Andy Pachikerl, Ph.D Introduction Anaplasma platys (formerly Ehrlichia platys) is a Gram negative, non-mobile, pleomorphic bacterium, belonging to the Anaplasmataceae family, which has been speculated, but not conclusively demonstrated, to be transmitted by Rhipicephalus sanguineus, known as the “brown dog tick” (Simpson et al. 1991). Anaplasma platys is an obligate intracellular microorganism, which appears to parasitise dog platelets exclusively, causing a Canine Vector-Borne Disease (CVBD) named Infectious Canine Cyclic Thrombocytopenia (ICCT) (Cardoso et al. 2010) due to the thrombocytopenia that relapses every 7-14 days (Harrus et al. 1997). Since its first identification in Florida (Harvey et al. 1978), A. platys infection has been reported in several countries around the world, including the United States, China, Thailand, India, Japan, Venezuela, Brazil, Chile, Israel and Australia (Abarca et al., 2007, Abd Rani et al. 2011, Brown et al. 2001, Cardozo et al. 2009, French et al. 1983, Hua et al. 2000, Inokuma et al. 2001, Suksawat et al. 2001). With regard to Europe, the presence of A. platys has been reported in France, Italy, Spain, Greece, Portugal, and Croatia and in 2 dogs imported in Germany (Beaufils et al. 2002, De La Fuente et al. 2006, Dyachenko et al. 2012, Ferreira et al. 2007, Kontos et al. 1991). Despite the increasing interest in Vector Borne Pathogens (VBPs) affecting dogs in Italy (Dantas‑Torres et al. 2012), the infection by A. platys is poorly documented and considered to be sporadic throughout the country. Nonetheless, A. platys has been serologically and molecularly detected in dogs from Southern regions (Sicily, Apulia and Abruzzo), mostly in co-infection with other VBPs (16, 17, 35, 37,39). Moreover, the DNA of this pathogen has also been found in R. sanguineus ticks by PCR (Sparagano et al. 2003). Diagnosis Blood tests and a urinalysis are the main diagnostic tools for anaplasmosis. The blood tests usually include a complete blood count, blood smear evaluation, biochemistry panel, serology to look for antibodies, and polymerase chain reaction (PCR) assays. If the dog is lame, radiographs and analysis of joint fluid are usually included. Among the current available diagnostic methods for detection of A. platys infection, the most used include morulae identification in the blood smears, antibody detection and DNA amplification by PCR (Otranto et al. 2010). Demonstration of the intra-platelet inclusion bodies of A. platys on blood or buffy-coat smears commonly represents the first diagnostic approach in A. platys infection, especially during the acute phase of disease. On the basis of the study described in this article, an accurate, light microscopy analysis of the stained blood smears appears to be a reliable method to point the diagnosis in the direction of A. platys infection, as it allowed platelet cytoplasmic inclusions resembling A. platys morulae to be detected and acute infection to be suspected in all 3 clinical cases. However, a definitive detection of the organisms in blood films may be difficult and cannot be considered a reliable diagnostic method in the chronic phase of the infection due to the cyclic course of bacteraemia, the rarely found parasitaemia and the fairly frequent presence of a very low number of infected platelets (Harrus et al. 1997, Otranto et al. 2010). Furthermore, it should be considered that inclusion bodies within platelets may be present and related to platelet activation during inflammation and E. canis infection and, thus, misdiagnosed as A. platys morulae (Ferreira et al. 2007). Serological methods, such as IFAT, were not taken into account in the diagnostic approach of A. platys infection because they are uncommonly applied, due to the difficulty in obtaining A. platys-infected platelets to use as antigen (A. platys has not yet been cultured) (Lai et al. 2011, Martin et al. 2005) and the possible false-positive results linked to the serologic cross-reactivity between organisms belonging to the same sero-group (e.g. A. phagocytophilum). Recently, a simple qualitative in-clinic Enzyme Linked Immunosorbent Assay (ELISA), the Snap®4Dx Plus (IDEXX Laboratories, Westbrook, ME, USA) was developed in order to identify antibodies against A. platys, as well as to detect Dirofilaria immitis antigen and antibodies for further VBPs e.g. A. phagocytophilum, E. canis, E. erwiingi, B. burgdorferi. Similarly, some rapid tests developed by bioguard or biogen also detect the antibodies against A. platys and other VBP. Thess rapid tests gained favour among small-animal practitioners due both to its ease of use and its accuracy; however, is not able to distinguish between A. phagocytophilum and A. platys. Moreover, the presence of anti-A. platys antibodies does not mean clinical infection, but rather exposure to the infectious agent (Martin et al. 2005). Recently, more specific and sensitive strategies focusing on molecular methods based on PCR approaches were employed (Eddlestone et al. 2007, Ferreira et al. 2007, Inokuma et al. 2002, Lai et al. 2011, Martin et al. 2005) to enable the diagnosis of active cases of A. platys infection, which would otherwise have gone undetected due to low‑sensitivity of microscopy and the low-specificity of the serological diagnosis. It has been demonstrated that PCR is positive even in the case of low-level parasitaemia (Otranto et al. 2010). Several PCR assays were optimized to allow for accurate identification of A. platys infection in dogs using different targets (16S rRNA, p44, groESL, gltA). Therefore, the PCR test, confirmed by a sequence analysis of amplicons, is the most reliable diagnostic test for this pathogen to date (Aguirre et al. 2006, De La Fuente et al. 2006, Gaunt et al. 2010). Treatment Treatment includes antibiotics, pain relievers, and anti-inflammatory drugs. Doxycycline is the most used antibiotic. Most dogs respond within one to two days after they first take doxycycline. Other antibiotic options are tetracycline or minocycline. Analgesia and anti-inflammatory drugs may be needed for joint pain. Let your veterinarian choose the anti-inflammatory, rather than choosing and dosing it yourself, because dogs metabolize these medicines differently than humans do. Your veterinarian will have the most appropriate medication. Disease Prevention Appropriate tick control is critical to preventing this disease. Preventing ticks from
Chlamydophila felis: A Unique Bacteria Causing Diseases to Felines
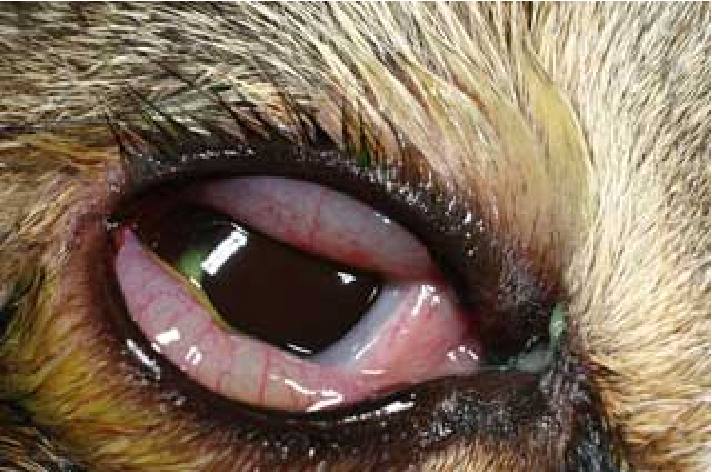
Chlamydophila felis: A Unique Bacteria Causing Diseases to Felines Maigan Espinili Maruquin Structure and Replication The chlamydiae is unique obligate intracellular bacteria. The Chlamydophila felis is a Gram- negative and rod- shaped coccoid bacterium however the cell wall lacks peptidoglycan (Gruffydd-Jones, Addie et al. 2009). It has two morphologically distinct structures. The (1) EB or elementary body is metabolically inert infectious, round and small (~0.3 μm), and is responsible for its survival in extracellular environment with its ‘spore-like’ form with a rigid cell wall. It holds the central and dense nucleoid. Whereas, the other form (2) RB or the replicative but noninfectious reticulate body which is larger (~1 μm) than the EB. It has cross-linked membrane proteins which makes it structurally flexible and osmotically fragile. It contains RNA and diffuse and fibrillary DNA, allowing intracellular replication, nutrient uptake and transportation, protein synthesis and other metabolic activities (Bedson and Bland 1932, Moulder 1991, Nunes and Gomes 2014). The chlamydiae is unique for its biphasic developmental cycle of 30–72 hours (Nunes and Gomes 2014). This bacteria, during its intracellular life, stays in a parasitophorous vacuole, or inclusion to acquire the nutrition it needs. (Hybiske and Stephens 2007). First, the EB attaches and enters the host cell which leads to formation of vacuole. Inside the inclusions, the EB differentiates from the RB. The RB then replicates via binary fission (Borges, V. et. al., 2013) (Nunes and Gomes 2014). The inclusions then expand while RB undergoes transition or conversion back to EB. Finally, the bacteria is released through host cell lysis or via extrusion (Hybiske and Stephens 2007, Nunes and Gomes 2014). Fig. 01. The unique biphasic developmental cycle of Chlamydiae (Source: https://www.researchgate.net/figure/Chlamydia-undergo-a-unique-biphasic developmental-cycle-The-infectious-form-of_fig3_268229035 ) Chlamydophila felis Epidemiology The Chlamydiaceae is reported to have cause animal infection often indirectly and associated with other pathogens (Schautteet and Vanrompay 2011, Nunes and Gomes 2014). The Chlamydophila felis grows in the cytoplasm of epithelial cells and produces inclusion bodies (Halánová, Sulinová et al. 2011). The C. felis requires close contact between cats to transmit while ocular secretions are considered the most important body fluid for the infection. The disease caused by the C. felis is common in multi- cat environments (Wills JM et al., 1987)(Gruffydd-Jones, Addie et al. 2009) while it is also frequently associated with conjunctivitis (WILLS, HOWARD et al. 1988, Gruffydd-Jones, Addie et al. 2009). Despite the low zoonotic potential, possible exposure to the C. felis is through handling of infected cats, by contact with their aerosol and also via fomites (Baker 1942, Halánová, Sulinová et al. 2011). Reports in culture and PCR (Sykes, Anderson et al. 1999, Sykes 2005) showed that C. felis most likely to infect cats less than a year of age and less likely for cats age 5 years above. There is no strong breed or sex preference and prevalence of asymptomatic cases are low (Sykes 2005). Clinical Signs/ Pathogenesis The C. felis is known to cause conjunctivitis associated with severe swelling of the lid, mild rhinitis, ocular and nasal discharges, fever, and lameness (Masubuchi, K, et al. 2002)(TerWee, Sabara et al. 1998, Rodolakis and Yousef Mohamad 2010). In kittens, chlamydiosis most commonly cause pneumonia and conjunctivitis (TerWee, Sabara et al. 1998, Yan, Fukushi et al. 2000)( Sykes, J. E., 2001)(Halánová, Sulinová et al. 2011) and can cause disease to adults, too (Sykes 2005, Halánová, Sulinová et al. 2011). While C. felis affects conjunctival epithelial cells, natural transmission occurs by close contact with other infected felines, aerosols, and fomites with approximately 3 to 5 days of incubation period (Sykes 2005, Gruffydd-Jones, Addie et al. 2009). Generally, conjunctival shedding ceases at around 60 days after infection, however some cats may carry persistent infection (O’Dair HA , et al, 1994; Wills JM., 1986;) (Sykes 2005, Gruffydd-Jones, Addie et al. 2009). Due to the reported chlamydial conjunctivitis in the rectal and vaginal excretion from cats, intestinal and reproductive tracts were considered sites for the persistent infections (Wills JM., 1986)(Sykes 2005). On the other hand, findings of the C. felis were also in lung, spleen, liver, kidney and peritoneum of cats (Dickie CW, Sniff ES, 1980; Hoover EA, 1980) (Baker 1944, Masubuchi, Nosaka et al. 2002, Sykes 2005). Other microorganisms may coinfect C. felis. Felines infected by C. felis show clinical signs including: sneezing, transient fever, inappetence, weight lost, nasal discharge, vaginal discharge, lameness and lethargy (Halánová, Sulinová et al. 2011). Unilateral ocular disease may appear during the first day or two which progresses to bilateral. Discharge in the ocular is watery which becomes mucoid or mucopurulent while chemosis can be observed in the conjunctiva. However, although the cats show symptoms after infection, they mostly still continue to eat (Gruffydd-Jones, Addie et al. 2009). Fig. 2. A young cat from a multi- cat household showed chlamydial conjunctivitis (Heinrich, C., 2017). (Source: https://www.semanticscholar.org/paper/Bacterial-conjunctivitis-Chlamydophila-felis-Heinrich/12a1d5eee2fbc8121665d94441ff698531c32868 ) Diagnosis Although the use of indirect immunofluorescence can be used to detect the serum antibody titer, this method should be used after a diagnosis of considerable rise in antibody titer (Sykes 2005, Gruffydd-Jones, Addie et al. 2009). On the other hand, cell culture is considered to be the golden standard in diagnosing chlamydial infections (Pointon AM, et al., 1991) (Wills JM, et. al., 1988) (Sykes 2005). The cell culture technique uses fluorescent antibodies in detecting inclusions. However, cell culture isolation is a demanding, time-consuming and expensive technique while sensitivity of the culture may vary depending on the equipment being used and the technical expertise (Sykes 2005). Further, Giemsa staining can be used for inclusions but this causes confusion with other basophilic inclusions (Gruffydd-Jones, Addie et al. 2009) Also, conjunctival smears can be Giemsa stained to look for inclusions, but chlamydial bodies are easily confused with other basophilic inclusions (Streeten BW, Streeten EA, 1985) (Gruffydd-Jones, Addie et al. 2009) and inclusions are often seen only on early infection, and at times, they are not visible (Wills JM, 1986)(Sykes 2005). For a quicker, less expensive and more sensitive diagnosis than cell culture and
