Neoplasia: The Danger of Feline Leukemia Virus
Feline Leukemia Virus (FeLV) remains one of the most consequential retroviruses affecting domestic cats, not only due to its immunosuppressive effects but also its strong association with neoplastic diseases. Among FeLV-positive cats, neoplasia—particularly lymphoma—is a leading cause of mortality. 1. How does FeLV Causes Cancer? FeLV integrates its RNA into the host’s DNA, altering cellular […]
Urinary Tract Infections (UTIs) in Dogs: Why my…?

Urinary tract infections (UTIs) are common in dogs and can affect their comfort, health, and behavior. In this article, we will address the common questions that dog owners have regarding their dog urination. Early detection and treatment are essential to prevent complications such as kidney infections or bladder stones. 🔍 What is a UTI in […]
Unmasking the Spread: How Antibiotic Resistance Travels and Grows

Antibiotic resistance is no longer just a human healthcare issue—it’s a shared threat across both medical and veterinary fields. As veterinarians, we stand at the front lines, where responsible antibiotic use can make a critical difference. But how exactly does antibiotic resistance develop and spread? Let’s break it down. Step 1: Antibiotic Use in Animals […]
Von Willebrand’s Disease (vWD) in Dogs

Von Willebrand Disease (vWD) is the most common inherited bleeding disorder in dogs. It’s caused by a deficiency or dysfunction of a specific blood clotting protein called von Willebrand factor (vWF). This protein helps blood platelets stick together to seal broken blood vessels and stop bleeding. Without enough functional vWF, dogs with vWD can experience […]
Why Are Cats More Prone to CKD (Chronic Kidney Disease)?

Chronic kidney disease (CKD) can affect cats at any age but is more commonly diagnosed in middle-aged and senior cats, typically those over seven years old. There are several reasons that causes CKD, which are: 1. Natural Aging Process: Unlike dogs, whose kidneys tend to fail due to infections or inherited conditions, CKD in cats […]
Protecting Your Pup: Canine Distemper

Canine distemper is a highly contagious and potentially fatal viral disease caused by the Canine Distemper Virus (CDV), a member of the Paramyxoviridae family. Despite advances in vaccination, this disease remains a significant threat, particularly in unvaccinated dogs and wildlife populations such as foxes, wolves, raccoons, and skunks. Pathogenesis CDV primarily targets the respiratory, gastrointestinal, […]
Protecting Dogs from the Threat of Leptospirosis

Leptospirosis is a zoonotic bacterial disease caused by pathogenic spirochetes of the genus Leptospira. It affects a wide range of mammals, including domestic animals, wildlife, and humans. Leptospira are aerobic, gram-negative spirochetes characterized by their corkscrew-like motility. These bacteria are slow-growing and can survive for weeks to months in warm, moist environments such as […]
What is the Danger of AMR in animals?
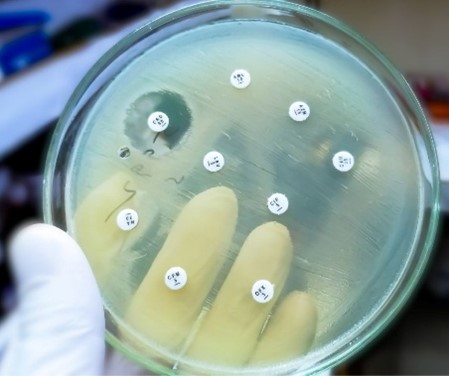
Antimicrobial resistance (AMR) occurs when microorganisms—such as bacteria, viruses, fungi, and parasites—adapt to withstand medications designed to eliminate them or inhibit their growth. This phenomenon reduces the effectiveness of treatments, making infections more difficult to control and increasing the risk of disease transmission, severe illness, and mortality. For veterinarians, understanding AMR is essential due to […]
WHAT ARE THE FACTORS THAT AFFECT “ANTIBIOTIC RANK”?

Factors Influencing the Ranking of Veterinary Antibiotics Resistance Potential One of the most critical factors in ranking veterinary antibiotics is their potential to promote antibiotic resistance. Drugs like fluoroquinolones and third-generation cephalosporins are closely monitored because of the risk that resistant strains can transfer from animals to humans through food consumption or direct contact. Spectrum […]
Feline Herpesvirus 1 (FHV-1) Infection

Bioguard Corporation Feline herpesvirus-1 (FHV-1) is a common viral infection in cats that primarily affects the upper respiratory system. It is a major cause of feline viral rhinotracheitis (FVR), which presents symptoms like sneezing, nasal discharge, and conjunctivitis (eye inflammation). FHV-1 is highly contagious among cats and is spread through direct contact with infected saliva, […]

