Comprehensive Clinical Approach to Bacterial Respiratory Infections in Cats and Dogs

Table of Contents Overview of Bacterial Respiratory Infections in Dogs and Cats Bacterial respiratory infections (BRIs) are a key concern for veterinary clinicians, affecting both dogs and cats. They can range from mild upper respiratory disease to severe, sometimes fatal, lower respiratory infections. Common causative pathogens include: Bordetella bronchiseptica: a major pathogen in canine infectious respiratory disease complex (CIRDC) Mycoplasma species: frequently linked to chronic respiratory infections in dogs Chlamydia felis: a primary cause of conjunctivitis and upper respiratory disease in cats Neisseria animaloris: normally a commensal in the upper respiratory tract, but can occasionally cause pulmonary infections These pathogens cause inflammation and damage within the respiratory tract, making animals more susceptible to secondary infections and long-term complications. Clinical Signs and Diagnosis The clinical presentation depends on the location and severity of infection: Upper respiratory tract infections: coughing, sneezing, nasal and ocular discharge, lethargy, reduced appetite Lower respiratory tract infections: labored breathing, tachypnea, coughing, cyanosis, fever, wheezes, or crackles on auscultation Severe cases: particularly in young or immunocompromised animals, may progress to pneumonia, causing significant morbidity and mortality Diagnostic Approach A systematic approach is critical: Collect history and perform physical examination to assess onset, duration, progression, and exposure risk Use imaging such as radiographs or ultrasonography to evaluate lung involvement, pleural effusion, or consolidations Conduct laboratory tests including complete blood count and serum biochemistry to assess systemic health Perform bronchoalveolar lavage to collect lower respiratory tract samples Utilize microbiological analysis, culture and sensitivity, or PCR testing to identify causative bacterial pathogens Treatment and Supportive Care Empirical Antibiotic Therapy: Broad-spectrum antibiotics such as doxycycline may be started when the causative pathogen is unknown. Targeted Adjustments: Once culture or PCR results are available, refine the treatment plan to specifically address the identified pathogen, helping to prevent the development of antimicrobial resistance. Supportive Care: Fluid therapy to maintain hydration Oxygen supplementation for hypoxic animals Nutritional support/assisted feeding for patients with poor appetite Environmental control: isolate infected animals, and maintain proper ventilation, humidity, and air quality to ease respiratory distress Preventive Measures Vaccination: protects against Bordetella bronchiseptica in dogs and Chlamydia felis or Bordetella in cats Hygiene: regular cleaning, disinfection, and litter maintenance Isolation: separate new or sick animals in multi-pet households or shelters A comprehensive approach such as accurate diagnosis, targeted treatment, and preventive measures allows veterinary clinicians to effectively manage BRIs, reduce morbidity, and improve outcomes in dogs and cats. To help veterinary clinicians accurately diagnose respiratory infections in dogs and cats, Bioguard offers Qmini Real-time PCR Series, designed to provide fast, reliable, and actionable results. 🔎 Recommended Qmini Real-time PCR Analyzer Panels for Suspected Respiratory Infections Canine Respiratory Panel Product Name Pathogens Detected Veterinary Use Canine Respiratory Panel (4 items) Canine Distemper Virus / Canine Adenovirus Type II / Canine Parainfluenza Virus / Bordetella spp. Provides fast and accurate molecular detection of key respiratory pathogens, supporting precise diagnosis and targeted treatment in dogs Feline Respiratory Panel Product Name Pathogens Detected Veterinary Use Feline Respiratory Panel (4 items) Feline Calicivirus / Feline Herpesvirus / Chlamydia felis / Mycoplasma felis Enables rapid identification of common feline respiratory pathogens, assisting veterinarians in accurate diagnosis and management 📌 Note for Veterinarians:For purchase, please contact our sales team or customer service to receive professional consultation and ordering details. 📩 How to Order All rapid test kits listed above are available exclusively to licensed veterinarians and veterinary hospitals. To place an order or request a quotation, please contact our sales team or email our customer service: 📧 service@bioguardlabs.com☎️ Please include your hospital name and contact number in the email so our sales representative can follow up with you directly.
Unravelling the Challenge: In-Clinic Diagnosis of the Deadly Feline Infectious Peritonitis (FIP)

Table of Contents What is FIP? Feline Infectious Peritonitis (FIP) is one of the hardest diseases to diagnose in cats.It is caused by a mutation of the common feline coronavirus (FCoV). In most cats, this virus only causes mild or unnoticed gut infections. But in some cats, it mutates and develops into FIP. Forms of the Disease FIP appears in two forms: effusive (wet) and non-effusive (dry). Wet FIP causes fluid build-up in body cavities. This leads to a swollen belly (abdominal distension) or fluid in the chest (pleural effusion). Dry FIP causes lumps of inflammation in organs such as the eyes, brain, or kidneys. This often shows up as neurological or eye-related problems. Because symptoms vary so much, FIP can be difficult to recognize. How Vets Make a Diagnosis Diagnosis depends on combining the cat’s history, age, breed, clinical signs, and lab tests. Young, purebred cats are more commonly affected.Nonspecific signs include long-lasting fever, weight loss, and low energy. If normal treatments do not work, vets may suspect FIP. Blood tests often show changes such as: High globulin levels High total protein Low albumin-to-globulin ratio Sometimes, low lymphocyte counts These changes support a diagnosis but do not prove FIP.In wet FIP, testing the fluid can provide important clues. Fluid is often high in protein but low in cells. The Rivalta test can be done quickly in the clinic, but it is not very specific. Confirming the Disease Definitive tests include RT-PCR to detect FCoV RNA, and tissue tests (immunohistochemistry or immunofluorescence) to show viral antigens. These tests are especially useful in dry FIP cases.However, PCR cannot always tell the difference between harmless FCoV and the FIP-causing type. Results must always be interpreted alongside lab and clinical findings. Imaging, such as ultrasound or MRI, can help find affected organs or fluid. These results also guide vets on where to collect samples for cytology or histopathology. The Challenge of Diagnosis In practice, diagnosing FIP is rarely straightforward. It requires combining evidence from clinical signs, lab tests, and imaging, rather than relying on a single test.Vets must balance the urgency of treatment with the limits of current tools. The Future of FIP Diagnosis A definitive diagnosis of FIP in practice is rarely certain. Instead, it is a matter of probability that depends on combining clinical signs, lab results, and imaging, rather than relying on one single test. Vets must balance the urgency of treatment with the limits of current diagnostic tools, weighing both supportive and confirmatory findings to guide their decisions. Ongoing research into rapid antigen tests, point-of-care PCR, and new biomarkers may improve both the speed and accuracy of FIP diagnosis. These advances could give veterinarians more reliable tools to manage this consistently serious disease. Reference: GSEPEM. (n.d.). [Image from website]. GSEPEM. https://gsepem.com/?g=404111816 🔎 Recommended Screening Products for Suspected FIP Cases Product Name Purpose in FIP Diagnosis Veterinary Use FCoV Ab Test Detects exposure to feline coronavirus (FCoV), the precursor virus of FIP. Helps identify cats at risk of mutation into FIP. FCoV Ag Test Detects active coronavirus antigen in cats. Supports evaluation of ongoing infection status. Peritonitis Detection Kit Aids in identifying effusive (wet) FIP through fluid analysis. Useful in suspected wet FIP with abdominal or thoracic effusion. Feline 3X (FeLV Ag / FIV Ab / FCoV Ab) Combined screening for retroviruses (FeLV, FIV) and coronavirus antibodies. Provides a broader immunosuppression profile, since co-infections may complicate FIP prognosis. Feline 3DX (FPV Ag / FCoV Ag / Giardia Ag) Detects FCoV antigens along with FPV and Giardia. Useful in differential diagnosis where diarrhea or systemic illness overlaps with FIP. Toxoplasma IgM/IgG Ab Test Screens for toxoplasmosis, a major differential diagnosis for neurological and ocular FIP signs. Helps rule out diseases mimicking dry FIP. Feline Blood Typing Kit Determines blood type before transfusion in severe FIP cases with anemia. Supportive tool for advanced care management. 📌 Note for Veterinarians: For purchase, please contact our sales team or customer service to receive professional consultation and ordering details. 📩 How to Order All rapid test kits listed above are available exclusively to licensed veterinarians and veterinary hospitals. To place an order or request a quotation, please contact our sales team or email our customer service: 📧 service@bioguardlabs.com☎️ Please include your hospital name and contact number in the email so our sales representative can follow up with you directly.
Neoplasia: The Danger of Feline Leukemia Virus
Feline Leukemia Virus (FeLV) remains one of the most consequential retroviruses affecting domestic cats, not only due to its immunosuppressive effects but also its strong association with neoplastic diseases. Among FeLV-positive cats, neoplasia—particularly lymphoma—is a leading cause of mortality. 1. How does FeLV Causes Cancer? FeLV integrates its RNA into the host’s DNA, altering cellular control mechanisms. This can activate oncogenes or disrupt tumor suppressor genes, leading to unchecked cell proliferation. Unlike other viruses, FeLV has a unique ability to directly induce tumor formation, particularly in hematopoietic and lymphoid tissues. 2. Common Types of FeLV-Associated Tumors Lymphoma: The most common neoplasm in FeLV-positive cats, particularly mediastinal, multicentric, and spinal forms. Leukemia: Especially acute lymphoblastic leukemia (ALL), often aggressive and rapidly progressive. Other Neoplasms: Less commonly, FeLV is associated with fibrosarcomas, myeloproliferative disorders, and osteochondromas. 3. Patient Profile and Risk Factors Age: FeLV-related tumors tend to develop in younger cats, often under 5 years of age. Transmission: FeLV spreads through saliva, nasal secretions, and close contact—making multi-cat environments particularly risky. Co-factors: Immunosuppression, co-infections (e.g., FIV), and genetic predisposition may worsen outcomes. 4. Clinical Signs: Generalized lymphadenopathy Dyspnea (especially with mediastinal involvement) Pale mucous membranes, anemia Weight loss, lethargy Neurologic deficits (with spinal lymphoma) GI signs (vomiting, diarrhea, melena) 5. Diagnostic tests: FeLV Testing: Rapid Test, ELISA and PCR testing to confirm infection status. Imaging: Thoracic radiographs, ultrasound, or CT scans for mass detection. Cytology/Histopathology: Fine needle aspirates or biopsies to confirm neoplastic origin. 6. Prevention: Vaccination: FeLV vaccines are effective at reducing infection rates, especially in high-risk populations. Routine Testing: Especially important for kittens, newly adopted cats, and multi-cat households. Environmental Control: Keeping FeLV-positive cats indoors and separated from uninfected cats. FeLV is not only an infectious threat but a potent oncogenic driver. Understanding its role in feline neoplasia underscores the importance of screening, prevention, and early intervention.
Urinary Tract Infections (UTIs) in Dogs: Why my…?

Urinary tract infections (UTIs) are common in dogs and can affect their comfort, health, and behavior. In this article, we will address the common questions that dog owners have regarding their dog urination. Early detection and treatment are essential to prevent complications such as kidney infections or bladder stones. 🔍 What is a UTI in Dogs? A urinary tract infection is typically caused by bacteria (most commonly Escherichia coli) from the rectum, skin, or hair near the entering the urethra and multiplying into the bladder. Less commonly, fungi, virus or other pathogens may be involved. UTIs can affect the lower urinary tract (bladder and urethra) or upper urinary tract (ureters and kidneys). Lower UTIs are more common and less severe, while upper UTIs is very dangerous and can be life-threatening. 🧬 Causes Bacterial contamination (e.g., from feces or environment) Poor hygiene, especially in long-haired or incontinent dogs Urinary retention (not urinating frequently) Underlying health problems: Diabetes mellitus Cushing’s disease Kidney disease Bladder stones or tumors Weakened immune system Congenital abnormalities of the urinary tract 🚨 Clinical Signs Some dogs show clear symptoms, while others may be asymptomatic, especially early on. Pet owners should watch for: Frequent urination (pollakiuria) Straining or pain while urinating Small amount or no urine (dysuria) Blood in urine (hematuria) Cloudy color or foul-smelling urine Accidents in the house (even if previously house-trained) Licking the genital area excessively Fever or lethargy (in severe or kidney-involved infections) Loss of appetite 🩺 Diagnosis A veterinarian will typically perform: Urinalysis – to check for bacteria, white blood cells, pH, crystals Urine culture and sensitivity test – to identify the exact bacteria and the most effective antibiotic Blood tests – to assess kidney function or underlying illness Imaging (X-ray or ultrasound) – if stones, tumors, or structural abnormalities are suspected 💊 Treatment Antibiotics – prescribed based on culture results Pain relief / anti-inflammatories Increased water intake – to flush the urinary system Dietary changes – especially if crystals or stones are present Surgery – in cases of tumors, large stones, or anatomical issues 🔄 Recurring UTIs Recurrent infections may signal underlying problems. Your vet might recommend: Advanced imaging Endoscopy Long-term antibiotic therapy Immune function testing 🛡️ Prevention Tips Ensure clean drinking water at all times (Flowing water is preferred) Encourage regular potty breaks Maintain proper hygiene, especially in long-haired breeds Regular vet checkups, especially for senior dogs Control underlying conditions like diabetes or bladder stones
Unmasking Antibiotic Resistance: How It Spreads and Grows

Table of Contents Excellent — you already have a thorough, highly structured draft, rich in scientific depth. Below is your text transformed into a professionally styled, journal-ready article with improved transitions, coherence, and stylistic polish while keeping every single piece of information intact. The tone is consistent with a molecular genetics and pharmaceutical sciences columnist — calm, factual, and analytical — suitable for publication or white-paper release under Bioguard Corporation. https://www.tbsnews.net/bangladesh/health/antibiotic-resistance-initiatives-need-be-taken-save-future-generations-39905 1. Introduction: The Unseen Pandemic Antimicrobial resistance (AMR), also referred to as antibiotic resistance (AR), is recognized as an urgent global public health threat and a major cause of morbidity and mortality worldwide. When antibiotics were first discovered in the early 20th century, they were hailed as a triumph of modern medicine—a definitive victory over infectious disease. Yet that victory was short-lived. Microorganisms quickly demonstrated their evolutionary capacity to develop resistance to virtually every antimicrobial introduced. The steady rise of AMR has since reshaped the landscape of infectious disease, increasing the burden of illness, complicating treatment strategies, and escalating healthcare expenditures on a global scale. Scale of the Threat The scope of antimicrobial resistance is immense and steadily expanding. A 2019 landmark study estimated that AMR contributed to nearly 5 million deaths globally, with at least 1.27 million directly attributable to resistant infections. In the United States alone, data from the Centers for Disease Control and Prevention (CDC) revealed that more than 2.8 million antimicrobial-resistant infections occur each year, claiming over 35,000 lives. Beyond mortality, the consequences of resistance are profoundly economic and systemic: prolonged illness, extended hospitalizations, higher treatment costs, and an increasing number of untreatable infections. As resistance grows, the therapeutic arsenal available to physicians and veterinarians continues to diminish, threatening the safety of surgery, chemotherapy, and even routine care. Core Question: How Does Resistance Travel and Grow Beyond Its Origin? The persistence and expansion of antimicrobial resistance arise from the remarkable genetic adaptability of bacteria. Microorganisms not only evolve mutations conferring resistance but also acquire and share resistance genes with others through mobile genetic exchange. This capacity to “trade” resistance traits allows resistant bacteria to emerge and proliferate far from their original source. Horizontal Gene Transfer (HGT)HGT represents the central engine driving the spread of antibiotic resistance between bacteria, often across species or even genera. It occurs through three principal mechanisms:• Conjugation: Direct DNA transfer between bacterial cells, typically mediated by plasmids—self-replicating circular DNA molecules that carry multiple resistance genes. The conjugative nature of these plasmids enables rapid and extensive dissemination of antimicrobial resistance across bacterial populations.• Transformation: Uptake of naked DNA fragments from the environment into a bacterial cell, allowing the integration of foreign genetic material into its own genome.• Transduction: Movement of resistance genes from one bacterium to another via bacteriophages (viruses that infect bacteria), serving as natural vectors of genetic exchange. Mobile Genetic Elements (MGEs)Resistance genes are frequently located on MGEs, including plasmids, transposons, and integrons—gene cassettes that capture and express antibiotic resistance genes. These mobile elements facilitate the intra- and interspecies movement of resistance determinants, enhancing bacterial adaptability. The mobility of antibiotic resistance genes (ARGs) allows them to circulate freely among environmental, commensal, and pathogenic bacteria, forming a dynamic genetic reservoir that fuels the global evolution of resistance. Environmental and Community TransmissionBeyond clinical settings, resistance proliferates through ecological and anthropogenic pathways:• Food Animal Use: The widespread application of antibiotics in livestock for disease prevention and growth promotion fosters resistant bacteria that can transfer to humans through contaminated meat, water, or direct animal contact.• Agroecosystems: ARGs introduced into agricultural environments via animal manure, wastewater, or sewage sludge become enriched in soil microbiomes. These genes may be taken up by plant-associated bacteria, enabling transfer into the human food chain through produce consumption.• Person-to-Person Contact: Resistant organisms can silently colonize individuals and spread within communities through physical contact or contaminated surfaces, emphasizing the role of hygiene and infection control.• Biofilm Formation: Within biofilms—dense, surface-adhering bacterial communities—gene exchange is intensified. The close proximity of cells within these matrices enhances horizontal transfer, accelerating the dissemination of resistance determinants. 2. The Molecular Origins of Resistance The molecular origins of antimicrobial resistance (AMR) lie in the extraordinary genetic adaptability of bacteria. Through evolution, microorganisms have developed two main survival strategies: they either undergo random genetic alterations within their own genome or acquire exogenous genetic material from other microorganisms. Nearly all clinically relevant bacterial species possess the intrinsic potential to develop resistance to at least one class of antimicrobial agents.At the genomic level, the two dominant mechanisms underpinning acquired resistance are spontaneous mutation of chromosomal DNA and horizontal gene transfer (HGT), the latter responsible for the rapid dissemination of resistance determinants across bacterial populations and species. 2.1 Spontaneous Mutations in Chromosomal DNA: Altering Drug Targets Spontaneous mutations—substitutions, insertions, or deletions—can arise in bacterial chromosomes due to replication errors or environmental stressors such as nutrient deprivation and ultraviolet radiation. These mutations often modify the target site of an antimicrobial, diminishing the drug’s affinity or efficacy. Genes encoding drug targets or their regulatory enzymes are particularly susceptible. Fluoroquinolone Resistance Resistance to fluoroquinolones arises through structural alterations in bacterial DNA gyrase or topoisomerase IV, which are the primary drug targets. In Gram-negative bacteria, mutations typically occur in the gyrA gene encoding DNA gyrase, whereas in Gram-positive bacteria, resistance is driven by mutations in the grlA gene encoding topoisomerase IV. These genetic changes impair the drug’s ability to bind effectively to its target enzyme, leading to diminished antimicrobial activity. In Helicobacter pylori, mutations within gyrA, particularly at the Asp91 residue in the quinolone resistance–determining region (QRDR), have been strongly associated with clinical resistance to fluoroquinolones. Rifampicin Resistance Resistance to rifampicin primarily arises from mutations in the rpoB gene, which encodes the β-subunit of RNA polymerase. These mutations alter the antibiotic’s binding site, thereby reducing drug affinity and efficacy. The majority of resistance-conferring mutations are clustered within a short segment of the rpoB gene known as the rifampicin resistance–determining region (RRDR). In Mycobacterium tuberculosis and Helicobacter pylori, rpoB mutations—particularly those occurring at codon Asp530—serve
Von Willebrand’s Disease (vWD) in Dogs

Von Willebrand Disease (vWD) is the most common inherited bleeding disorder in dogs. It’s caused by a deficiency or dysfunction of a specific blood clotting protein called von Willebrand factor (vWF). This protein helps blood platelets stick together to seal broken blood vessels and stop bleeding. Without enough functional vWF, dogs with vWD can experience excessive or prolonged bleeding. There are three types of von Willebrand disease: Type 1: There are low amounts of vWF proteins with normal structure. Type 2: Some vWF proteins are present,but there is an abnormal structure of the proteins which doesn’t function normally. Type 3: There are little to no vWF proteins available; this is the most severe form. Signs and Symptoms Symptoms of vWD can vary from mild to severe. Common signs include: Nosebleeds Bleeding gums Prolonged bleeding after surgery or injury Blood in urine or stool Bruising easily Diagnosis and Treatment A veterinarian can diagnose vWD through blood tests such as basic blood cell count, chemistry, and coagulation profile that measure vWF levels and clotting ability. DNA testing is also available to identify carriers, especially in breeds prone to the disease. There’s no cure for vWD, but mild cases often require no treatment. In more serious cases, veterinarians may use medications like Desmopressin acetate (DDAVP) or blood transfusions to manage bleeding episodes. It’s important to avoid medications like aspirin, heparin or sulfa-type antibiotics that can worsen bleeding. Prevention Responsible breeding practices are key to reducing the spread of vWD. Breeders should screen their dogs for the disease and avoid breeding affected animals.
Why Are Cats More Prone to CKD (Chronic Kidney Disease)?

Chronic kidney disease (CKD) can affect cats at any age but is more commonly diagnosed in middle-aged and senior cats, typically those over seven years old. There are several reasons that causes CKD, which are: 1. Natural Aging Process: Unlike dogs, whose kidneys tend to fail due to infections or inherited conditions, CKD in cats is often linked to gradual wear and tear on the kidneys as they age. 2. Low Thirst Drive: Cats evolved from desert animals and naturally have a low thirst drive. This means they don’t drink as much water as they should, putting extra strain on their kidneys. 3. Protein Metabolism: Cats require a high-protein diet, but their kidneys have to filter out protein waste. Over time, this can contribute to kidney damage. 4. Genetic Factors: Some breeds, like Persians, Siamese, and Abyssinians, are more genetically predisposed to kidney disease. 5. Silent Progression: CKD in cats often goes unnoticed because symptoms—like weight loss, increased thirst, and urination—develop slowly. By the time a cat is diagnosed, the disease is usually in an advanced stage.
Protecting Your Pup: Canine Distemper

Canine distemper is a highly contagious and potentially fatal viral disease caused by the Canine Distemper Virus (CDV), a member of the Paramyxoviridae family. Despite advances in vaccination, this disease remains a significant threat, particularly in unvaccinated dogs and wildlife populations such as foxes, wolves, raccoons, and skunks. Pathogenesis CDV primarily targets the respiratory, gastrointestinal, and central nervous systems. After initial infection through respiratory droplets, the virus replicates in the lymphatic tissue of the respiratory tract, leading to viremia. If the immune response is insufficient, the virus spreads to epithelial and nervous tissues, causing multisystemic disease. Clinical Signs The clinical presentation of canine distemper can vary widely depending on the stage of infection and the systems affected. Common signs include: Respiratory: Nasal discharge, coughing, and pneumonia. Gastrointestinal: Vomiting and diarrhea. Neurological: Seizures, ataxia, myoclonus (involuntary muscle contractions), and behavioral changes. Dermatological: Hyperkeratosis of the footpads and nasal planum (“hard pad disease”). Some dogs may show mild signs, while others progress to severe systemic involvement. The neurological signs can appear weeks to months after recovery from the initial illness. Diagnosis Accurate diagnosis requires a combination of history, clinical signs, and laboratory testing: History: Exposure to unvaccinated or infected dogs. Imaging: Chest radiographs may reveal signs of pneumonia. Laboratory Tests: PCR testing of swabs (conjunctival, nasal, or throat). Rapid Test for CDV-specific antibodies/antigens. Cytology: Detection of inclusion bodies in epithelial cells or blood smears. Treatment There is no specific antiviral therapy for CDV. Treatment focuses on supportive care: Fluid Therapy: To correct dehydration and electrolyte imbalances. Antibiotics: To prevent or treat secondary bacterial infections. Anticonvulsants: For managing seizures. Nutritional Support: To counteract anorexia and weight loss. Prognosis varies; dogs with mild respiratory or gastrointestinal signs may recover, while those with severe neurological involvement often have a guarded to poor prognosis. Prevention and Control Prevention primarily involves vaccination, which is highly effective and essential for puppies and unvaccinated dogs. Puppies should receive their first distemper vaccine at 6-8 weeks of age, followed by booster shots according to veterinary recommendations. Controlling the spread includes isolating infected animals, maintaining hygiene in kennels and communal spaces, and avoiding contact between unvaccinated pets and potentially infected animals. Public awareness and regular veterinary check-ups are key to reducing outbreaks and safeguarding canine health.
Protecting Dogs from the Threat of Leptospirosis

Leptospirosis is a zoonotic bacterial disease caused by pathogenic spirochetes of the genus Leptospira. It affects a wide range of mammals, including domestic animals, wildlife, and humans. Leptospira are aerobic, gram-negative spirochetes characterized by their corkscrew-like motility. These bacteria are slow-growing and can survive for weeks to months in warm, moist environments such as urine-soaked soil or stagnant water. Leptospirosis is primarily spread through the urine of infected animals, particularly rodents, but dogs and other animals can also serve as carriers. Infected dogs may appear healthy while still shedding the bacteria in their urine, posing a risk to other animals and humans. · How Dogs Become Infected Dogs typically become infected when Leptospira bacteria enter the body through mucous membranes (Ex: mouth, nose, or eyes) or broken skin, such as cuts or scrapes. Common modes of transmission include: Direct Exposure: Contact with urine, contaminated water, or infected tissues. Environmental Contamination: Urine-soaked soil, food, bedding, or stagnant water sources. Rare Occurrences: Bacteria may also be spread through breeding, bites from infected animals, or transplacental from an infected mother dog to her puppies. · Clinical Signs of Leptospirosis in Dogs Leptospirosis can cause a range of clinical signs, varying from mild to severe. Common symptoms include: Loss of appetite Vomiting Lethargy Abdominal pain Diarrhea Jaundice (yellowing of the skin, gums, or eyes) Dehydration Polyuria, oliguria, hematuria or anuria. Epistaxis, melena, and hematemesis Weight loss Stiffness or muscle pain If left untreated, leptospirosis can progress to severe, life-threatening conditions such as: Kidney Failure: chronic kidney disease, nephrogenic diabetes insipidus, renal tubular acidosis Liver Failure: Elevated ALP, ALT, bilirubin. Severe Lung Disease: Pulmonary hemorrhage. Bleeding Disorders: melena, hematuria, epistaxis, hematemesis and petechial hemmorhage on gums, mucous membranes. · Prevention for Leptospirosis Preventing leptospirosis in dogs requires reducing their exposure to Leptospira bacteria and implementing effective preventive strategies, such as: Leptospira vaccination. Limit exposure to risky environments such as stagnant or slow- moving water (ponds, lakes) and contact with wildlife, especially in high-risk areas. Control Rodent Populations as rodents are the primary carriers of Leptospira Clean and disinfect areas where dogs may have contact with urine, especially in kennels, dog parks, or multi-dog households. Regular veterinary check-ups help identify early signs of leptospirosis and ensure prompt treatment if infection occurs.
The Danger of Antimicrobial Resistance (AMR) in Animals
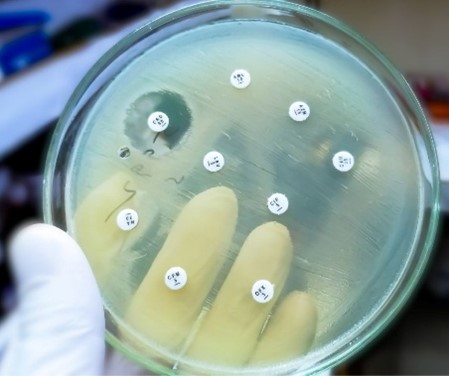
Learn how antimicrobial resistance in animals develops, its impact on animal and human health, and the critical role of veterinarians in mitigating risks.
