Feline Alpha-1-acid glycoprotein (AGP)
Feline Alpha-1-acid glycoprotein (AGP) Andy Pachikerl, Ph.D Introduction Alpha-1-acid glycoprotein (AGP) surges in cats’ blood when they fall in victim of feline infectious peritonitis (FIP), a lethal disease caused by feline coronavirus (FCoV). The diagnosis of feline infectious peritonitis (FIP) is often tough and not very viable at times. The clinical suspicion of FIP might be supported by the detection of effusions if any are present (Hartmann, et al., 2003; Paltrinieri, Parodi, & Cammarata, In Vivo Diagnosis of Feline Infectious Peritonitis by Comparison of Protein Content, Cytology, and Direct Immunofluorescence Test on Peritoneal and Pleural Effusions, 1999). The only way to conclusively test FIP to be positive is through histology followed by immunohistochemical or immunofluorescent detection of feline coronavirus (FCoV) within intralesional macrophages (Addie, Paltrinieri, & Pedersen, 2004; Barlough & Stoddart, 1990). Various studies suggested the implementation of biopsies for the confirmation of FIP in veterinary practices and it turns out to be quite useful with just a few downside (Alessia, Paltrinieri, Bertazzolo, Milesi, & Parodi, 2005). The application of biopsies in vivo is usually restricted due to anaesthetic risks especially via surgical biopsy and the relatively high percentage of unsuitable or falsely negative tru-cut or fine-needle aspiration biopsies (Alessia, Paltrinieri, Bertazzolo, Milesi, & Parodi, 2005). Serology and polymerase chain reaction techniques are not suitable for FIP diagnosis because they do not differentiate between the widespread low pathogenic FCoVs and the mutant pathogenic FCoV strains (Addie, Paltrinieri, & Pedersen, 2004; Herrewegh, et al., 1997). A previous study (Stoddart, Whicher, & Harbour, 1988) reported high levels of a1-acid glycoprotein (AGP) in cats with experimentally induced FIP. This finding was confirmed by another study, (Duthie, Eckersall, Addie, Lawrence, & Jarrett, 1997) which proposed the possible use of serum AGP as a diagnostic tool for FIP. Serum AGP is now widely used in diagnostic profiles for FIP.1 However, serum AGP levels increase in inflammatory disorders other than FIP (Duthie, Eckersall, Addie, Lawrence, & Jarrett, 1997; Kajikawa, Furuta, Onishi, Tajima, & Sugii, 1999; TerWee, Lauritzen, Sabara, Dreier, & Kokjohn, 1997; TerWee, et al., 1998), neoplasia (Correa, Mauldin, Mauldin, & Mooney, 2001), and asymptomatic but FCoV-positive cats. This lack of specificity limits the diagnostic potential of serum AGP as a diagnostic test for FIP. For more information on FIP and FCoV can be found in previously published report article (link). FIP clinical diagnosis through feline AGP AGP has been used extensively, particularly in Europe, as an indicator test for FIP. AGP was found almost a decade ago to be hyposialylated in cats with FIP, but not in normal cats or in cats with other pathologies (Fabrizio, Claudia, Alessia, Vanessa, & Saverio, 2004). This study confirmed that serum AGP is a powerful discriminating marker for FIP, but only when coupled with other high risk factors (Saverio, Giordano, Tranquillo, & Guazzetti, 2007). A Bayesian approach demonstrated that, when the pre-test probability of FIP was high based on history and clinical signs, moderate serum AGP levels (1.5–2 μg / mL) could discriminate cats with FIP from others. However, only high serum AGP levels (>3 μg / mL) were highly suggestive of FIP in cats with a low pre-test probability of disease (Saverio, Giordano, Tranquillo, & Guazzetti, 2007). Giori, et al. (2011) had shown specificity and sensitivity of several tests in 12 cats, four of which have the absence of FIP via histopathology and immunohistochemistry, and eight cats with FIP confirmed via histopathology and immunohistochemistry. Results from serum protein electrophoresis, analysis of effusions, anti-feline coronavirus serology, serum AGP concentrations and histopathology were then compared with the confirmed diagnosis. No concordance was found for serology and analysis of effusions, poor concordance was noted for histopathology, fair concordance for serum electrophoresis and perfect concordance for AGP. Their study proved that immunohistochemistry is always required to confirm FIP and, if immunohistochemistry is not feasible, they concluded that histopathology is not definitive, whilst elevated AGP concentrations might support the diagnosis of FIP. However, the small numbers of cats in this study make it difficult to validate such conclusions and the earlier study of Saverio, et al. (2007) is probably a more accurate assessment of AGP testing for FIP. Like most indirect tests for FIP, the positive predictive value increases with the number of other risk factors that are present. Saverio, et al. (2007) also investigated the levels of leukocyte bound AGP in normal cats and cats with diseases including FIP by flow cytometry using an anti-feline AGP antibody. A total of 32 healthy cats (19 feline coronaviruses seropositive), 13 cats with FIP (presumably all coronavirus seropositive) and 12 cats with other diseases (six coronaviruses seropositive) were studied. The proportion of cats with AGP-positive leucocytes in each group or in cats with different intensities of inflammatory response (as measured by CBC, serum electrophoresis and serum AGP levels) was compared. AGP positive leucocytes were found in 23% of cats; most were diseased, but a small number were healthy. AGP positive leukocyte staining was associated with inflammation and not with leucocytosis per se. Staining among healthy cats was unrelated to coronavirus antibody status. Cats with FIP were more likely to have positive staining leukocytes than healthy cats, but not as likely as cats with other diseases. It was concluded that AGP positive leucocytes are present in feline blood, especially during inflammation. Staining leukocytes for AGP binding do not appear to have any value over serum AGP testing, especially when considering the potential cost and effort involved in this method. A previous study by Paltrinieri, et al. (2007) showed positive correlation between AGP and FIP cats. In their study, they used 2 different groups of cats with FIP or non-FIP along with others contracted with other diseases such as FCoV. Their schematic experiment is as follows: Group 1. FIP group. This group was composed of 58 cats that had clinical signs and laboratory findings confirmatory of effusive FIP (n 5 53) or dry FIP (n 5 5). Haematology and serum biochemistry in these cats revealed nonregenerative anaemia, neutrophilia, lymphopenia, increased total
Breed-related disease: German Shepherd

The German Shepherd is a breed of medium to large-sized working dog that originated in Germany, Intelligent as it is versatile, this breed was originally developed in Germany to guard and herd a shepherd’s flocks. It has a double coat, which is comprised of a thick undercoat and a dense, slightly wavy or straight outer coat. Its hair, usually tan and black, or red and black, is medium in length and is shed all year round. Other rarer color variations include all-Black, all-White, liver and blue. The German Shepherd’s body is long-generally between 22 and 26 inches-in proportion to its height. This gives the dog strength, agility, elasticity and long, elegant strides. Because this watchful, self-assured breed is nearly unmatched in intelligence, German Shepherds excel in high-pressure jobs that require next-level problem solving, like search and rescue or police work. These extremely confident dogs are also keen observers and thinkers who have an uncanny ability to make decisions and problem-solve on the fly. They’re lauded for their courage, which is another trait that makes them a versatile working companion. Though German Shepherds might seem aloof around strangers, they bond easily with their families and are incredibly loving companions. The German Shepherd has an average lifespan of between 10 to 12 years. It is, however, susceptible to some serious health conditions like: Perianal Fistula: which is a disorder most commonly seen in German Shepherds. The disease is characterized by draining openings on the skin around the anus. Affected dogs may strain to defecate, have diarrhea or bloody stool and lick at the anal area frequently Megaesophagus: (from the Greek Mega meaning large) is a condition in which the esophagus (the tube that carries food to the stomach when we swallow) becomes limp and is not able to normally pass the food on its way to be digested. The type of megaesophagus that we see in German Shepherds is a congenital problem that a recent study found to correlate to chromosome 12. Affected dogs often begin to show signs, vomiting and regurgitation when they are weaned to a solid diet. Hip Dysplasia: Most people by now know about hip dysplasia. The hip joint is a ball and socket joint and hip dysplasia causes malformation of the components leading to instability. There can be abnormalities in either the ball or the socket (or both) and the chronic laxity causes abnormal wear and leads to osteoarthritis. Degenerative Myelopathy : is a neurologic disease and is a recessive genetic disorder in the German Shepherd Dog. Affected dogs are usually middle-aged or older patients and this disorder are difficult to distinguish from other causes of spinal cord compromise like intervertebral disc disease found commonly in many types of dogs. This genetic cause of weakness and paraplegia can only be positively identified postmortem with a histological exam of spinal cord tissue. Exocrine Pancreatic Insufficiency (EPI): This disorder of the digestive system is potentially life-threatening (particularly in its acute form) but often responds well to treatment. It is more common in some breeds than others and is frequently seen in German Shepherd Dogs. Sources: https://www.petmd.com/dog/breeds/c_dg_german_shepherd https://iheartdogs.com/ask-a-vet-what-are-5-important-health-concerns-for-german-shepherd-dogs/ Photo credit: https://en.wikipedia.org/wiki/German_Shepherd https://www.akc.org/dog-breeds/german-shepherd-dog
Breed-related disease: Siberian cat

The Siberian is a centuries-old landrace (natural variety) of domestic cat in Russia, and recently developed as a formal breed with standards promulgated the world over since the late 1980s. As befits a cat from northern Russia, the Siberian wears a magnificent fur coat that not only protects him from the elements but also gives him a glamorous appearance that belies his gentle good nature. At first glance, the Siberian resembles the Maine Coon and the Norwegian Forest Cat, but he is differentiated by having a more rounded body and head. He also stands out for his large yellow-green eyes, tufted ears and neck ruff. The Siberian coat comes in many colors and patterns, but brown tabbies seem to be most popular. The Siberian cat is highly affectionate with family and playful when they want to be. However, their exercise needs aren’t overly demanding, and they’re just as happy to snuggle up with their humans as they are to chase a laser toy–maybe even happier. In Russia, the phrase Siberian health is associated with vitality, longevity, and ability to stay healthy despite the frigid climate of the Siberian region. This saying is very true when it comes to Siberian cats. Siberians tend to be sturdy, healthy and, while being purebred cats, do not present the owner with too many health issues. However, there are some health problems typical for cats in general, and for Siberians in particular. If you own a Siberian cat or kitten or are only planning to adopt one, it’s best to know ahead what types of health issues you may encounter, and how to help your cat overcome them. Hypertrophic Cardiomyopathy : This is a heart condition in which the walls of the heart are thicker than they should be. Instead of benefiting from a stronger heart, this condition makes it more difficult for the cat to pump blood to the rest of the body. Kidney Disease (PKD): It is a genetic mutation that leads to the development of benign cysts in the cat’s kidneys and other organs. It is a hereditary disease that’s fairly common for Siberian cats. Gum Disease: Many Cat Owners overlook the importance of dental hygiene in their furbabies, but with this breed, regular teeth brushing is crucial. Hereditary Cancer: Cancer is by far most common in the white Siberian Forest Cats, and can be linked to a specific pedigree lineage of “Gesha Olenya Krasa” and “Dolka Olenya Krasa”. Cats of this descent are known to have cancer-causing genes , known as oncogenes. However, as in most other animals with oncogenes, the presence of the gene doesn’t necessarily guarantee the presence of cancer, and other factors may help prevent its manifestation such as a healthy diet and regular checkups. Urinary Tract Disease: Also referred to as Urinary Crystals, the condition involves the formation of stone-like minerals, crystals and organic matter and reside in the cat’s urinary tract. This covers anything from kidney stones to blockages to infections of the kidney. Although it’s not completely known whether it’s completely hereditary, it’s very common in the Siberian Cat. Sources: http://www.vetstreet.com/cats/siberian https://www.siberiancatworld.com/siberian-cats-health-problems/ http://aubreyamc.com/feline/siberian/ https: //www.madpaws .com.au / blog / siberian-cat / Photo credit: https://cattime.com/cat-breeds/siberian-cats#/slide/1 https://cats.lovetoknow.com/Siberian_Cats
Understanding FPV and its Threat to Our Cats
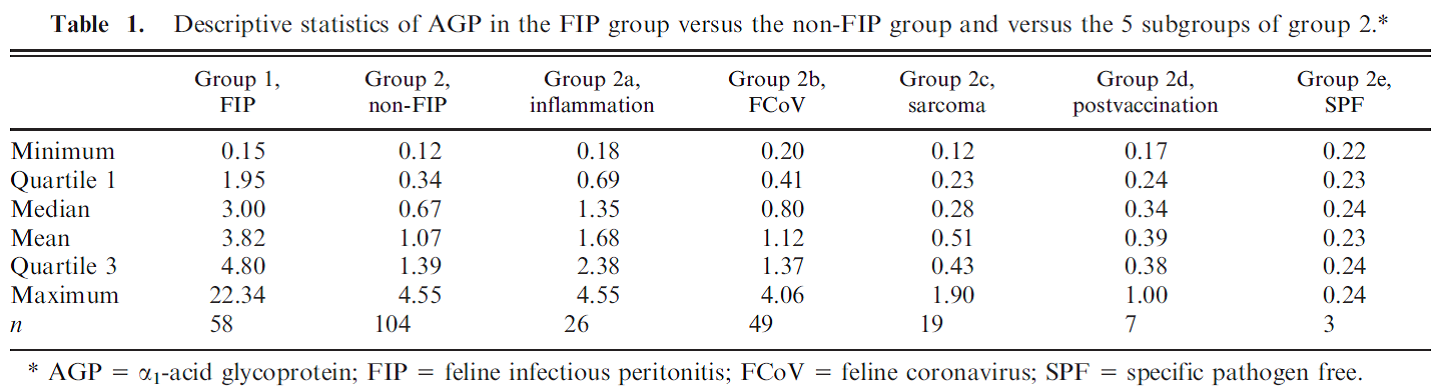
Understanding FPV and its Threat to Our Cats Maigan Espinili Maruquin The Feline Panleukopenia (FPL) is an important disease in cats. It is highly contagious and is often fatal to cats (Van Brussel, Carrai et al. 2019). This is caused by feline parvovirus (FPV; formerly FPL virus) and canine parvovirus (CPV), however, CPV infections in cats are uncommon (Barrs 2019). The FPL is also known to be the oldest known viral disease in cats wherein several epizootics that killed domestic cat populations in the 1800s could have been infected by FPV (Fairweather 1876, Barrs 2019) (Scott FW, 1987). Structure Fig. 01 A front view 60- meric assembly of FPV by Protein Data Bank in Europe containing 60 copies of Capsid protein VP1 ( https://www.ebi.ac.uk/pdbe/entry/pdb/1fpv ) The current taxonomic entity of FPV shares with CPV (Tattersall, 2006) wherein after crossing species barriers, CPVs evolved from FPV by acquiring five or six amino acid changes in the capsid protein gene (Truyen, 1999) (Appel, Scott et al. 1979, Black, Holscher et al. 1979, Osterhaus, van Steenis et al. 1980, Parrish 1990, Johnson and Spradbrow 2008, Stuetzer and Hartmann 2014, Barrs 2019). The causative agent FPV is a member of the genus Protoparvovirus in the family Parvoviridae with 5.2 kb long single stranded DNA genome, containing two open reading frames (ORFs): the first ORF encodes two non-structural proteins, NS1 and NS2; and the second ORF encodes two structural proteins, VP1 and VP2 (Reed, Jones et al. 1988, Zhou, Zhang et al. 2017). At first, FPV was thought not to infect cats (Truyen, Evermann et al. 1996). It replicates in thymus and bone marrow but not within the intestinal tract of dogs (Truyen and Parrish 1992, Truyen, Gruenberg et al. 1995). The pathway of viral entry into cells is not fully characterized, however through the feline transferrin receptor (TfR), FPV binds and uses the receptor to infect feline cells (Parker, Murphy et al. 2001, Hueffer, Govindasamy et al. 2003). However, CPV-2b and CPV-2c variants emerged, with only a single amino acid position different from CPV-2a, and infect cats both naturally and experimentally (Mochizuki, Horiuchi et al. 1996, Truyen, Evermann et al. 1996, Ikeda, Mochizuki et al. 2000, Nakamura, Sakamoto et al. 2001, Gamoh, Shimazaki et al. 2003, Decaro, Desario et al. 2011, Zhou, Zhang et al. 2017, Van Brussel, Carrai et al. 2019). FPV Infection The virus may be shed in feces even in the absence of clinical signs (subclinical infections), or before clinical signs are detected (Barrs 2019). The major portals of the FPV are the gastrointestinal (GI) tract and, less commonly, the respiratory tract. Generally, CPV is an uncommon cause of FPL and to date, no large-scale outbreaks of FPL have been confirmed to be caused by CPV (Barrs 2019). There were cases of indistinguishable CPV from FPV clinical signs in several cats (Mochizuki, Horiuchi et al. 1996, Miranda, Parrish et al. 2014, Byrne, Beatty et al. 2018, Barrs 2019). Moreover, coinfections of CPV and FPV were also reported in cats with clinical disease (Battilani, Balboni et al. 2011, Battilani, Balboni et al. 2013, Barrs 2019). The FPV can remain latent in peripheral blood mononuclear cells of healthy cats with high virus-neutralizing titers (Ikeda, Miyazawa et al. 1999, Miyazawa, Ikeda et al. 1999, Nakamura, Ikeda et al. 1999, Barrs 2019). The development of immunity of an unvaccinated cat to FPV is likely to increase with age (DiGangi, Levy et al. 2012). However, FPL mostly infects unvaccinated and incompletely vaccinated kittens. The age susceptibility correlates with the declining maternally derived antibodies (MDAs) as well as “the immunity gap” in incompletely vaccinated kittens (Barrs 2019). Clinical Signs/ Pathogenesis The FPV is resistant to heating (80C for 30 min) and low pH (3.0) (Goto, Yachida et al. 1974). Virions enter cells by endocytosis (Hueffer, Palermo et al. 2004). Viral DNA is released from the capsid and replicates through double-stranded RNA intermediates in the nucleus of the cell using the host’s DNA polymerase (Barrs 2019). It can be transmitted by the faecal-oral route and a contact with infected body fluids, faeces, or other fomites, as well as by fleas primarily spreads the virus. Viral replication primarily occurs in lymphoid tissue, bone marrow and intestinal mucosa in infected cats older than 6 weeks of age (Csiza, De Lahunta et al. 1971, Csiza, Scott et al. 1971, Parker, Murphy et al. 2001). Infection outcome ranges from subclinical to peracute infections with sudden death within 12 h (Stuetzer and Hartmann 2014). Initially, non-specific signs such as fever, depression, and anorexia during the acute stage (Addie, Jarrett et al. 1996). However, vomiting unrelated to eating occurs commonly and, less often, cats develop watery to haemorrhagic diarrhoea later in the course of disease, while some cats show extreme dehydration. Cats typically die of complications. Viral DNA can persist for long periods even after infectious virus has been lost, thus detection of DNA does not necessarily signify an active infection (Stuetzer and Hartmann 2014). Utero infection in early pregnancy can result in foetal death, resorption, abortion, and mummified fetuses while in later pregnancy may damage the neuronal tissue. The main clinical signs of FPV infection for new- born kittens include neurological, with ataxia, hypermetric movements and blindness, while some also shows signs of cerebellar dysfunction, forebrain damage (with seizures) with a range of severity and neurological signs. Although some kittens acquire MDAs, they can still get the virus for up to 2 months after birth (Csiza, Scott et al. 1971, Csiza, Scott et al. 1971, Stuetzer and Hartmann 2014). Infections occurring up to 9 days of age can also affect the cerebellum. Cats having mild cerebellar dysfunction may retain good quality of life. On the other hand, FPV can also cause retinal degeneration in infected kittens, with or without neurological signs (Percy, Scott et al. 1975, Stuetzer and Hartmann 2014). Diagnosis It is important to have the FPV detected early using accurate testing methods to prevent disease transmission
Canine HbA1c
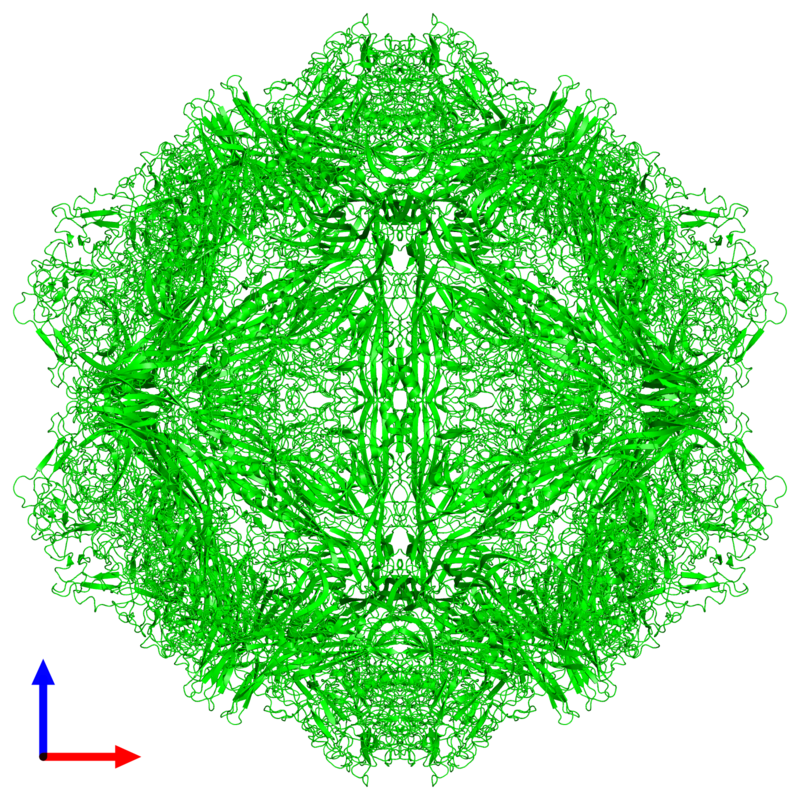
Canine HbA1c LIN, WEN-YANG (WESLEY), Ph.D HbA1c is a type of hemoglobin on which several monosaccharides such as glucose, galactose and fructose tend to bind with and exist in the bloodstream. The chemical linking process between sugar and hemoglobin is named glycation. HbA1c is actually an indicator of the beta-N-1-deoxy fructosyl on hemoglobin, which has been used as diagnostic measurement of long-term glycemic control for patients with diabetes mellitus. The increasing number of HbA1c in bloodstream represent the elevated plasma glucose level that usually indicating a poor diabetic management may lead to severer conditions. Due to the lifecycle of red blood cells is average four months, the HbA1c test could effectively present the real blood sugar degree in latest three months. In 1980, Wood and Smith first demonstrated the applicable method of examining canine diabetes with monitoring glycosylated haemoglobin. Later, Smith et al 1982, Mahaffey and Cornelius 1982, Dennis 1989, Jensen 1995 all confirmed this method is workable on diagnosing diabetic dogs. Molecular structure of HbA1c Figure 1 showed an aldimine linkage between glucose and hemoglobin (Figure1). Figure 1. Glycation process between Hemoglobin and glucose https://www.sciencedirect.com/science/article/pii/S0956566318304500 As we previously discussed that different monosaccharide would form different chemical structure with blood cells. Thus, while using cation exchange chromatography to separate Hemoglobin type A, different fractions would be separate out like HbA0, HbA1a, HbA1a2, HbA1b, and HbA1c. Fractions were named after eluting order (Figure 2). Figure 2. Different separated fractions of Hemoglobin type A with cation exchange chromatography https://www.sciencedirect.com/science/article/abs/pii/S0003269711000753 Glycated hemoglobin forming harmful factors in human body Highly reactive free radicals promote the formation of abnormal ferryl Hb (Fe4+-Hb), which enhance macrophage accumulation in blood vessel. Both macrophage accumulation and accumulation of glycated hemoglobin would elevate blood viscosity and slow down normal blood flow. Thus, atherosclerotic plaque would gradually occur in blood vessel. History of standardized HbA1c as diagnostic tool for diabetes Diabetes has become a serious health issue in entire world, due to over 220 million patients are suffering of it. Among all cases of diabetes, type 2 diabetes take majority part (90% to 95%). Besides, worsen type 2 diabetes could also cause extra complications, such as cardiovascular diseases, peripheral neuropathy, nephropathy, optic neuropathy, diabetic foot and even unto death. Several risks factor related to diabetes including high fat diet, obesity, smoking, elevated cholesterol levels, high blood pressure and lack of regular exercise. For preventing and relieving conditions of diabetes, taking healthy diet and regular exercise are important. In addition, diabetic patients’ blood sugar condition should be monitored regularly all the time. There are many clinical methods to evaluate glycemia like urine glucose, random or fasting plasma glucose etc. However, HbA1c is considered to be one of the most accurate and efficient method for measuring long-term blood sugar level (3 to 4 months). From 1894 to 1993, Diabetes Control and Complications Trial (DCCT) established the big data of diabetic patients’ HbA1c values to mean blood glucose resulted in making HbA1c a reliable index of mean blood glucose. However, DCCT haven’t standardized the HbA1c assay methods for labs and clinics. Later, the American Association for Clinical Chemistry (AACC) Standards Committee established a HbA1c Standardization Subcommittee to develop a plan for standardizing HbA1c assay that clinical laboratories could take advantage of it to perform precise glycemic evaluation and control. Now, the National Glycohemoglobin Standardization Program (NGSP) continue to develop reliable assays of HbA1c. Diagnosing canine diabetes with HbA1c 2018 American Animal Hospital Association suggested guidelines of diagnosis and assessment for animal diabetes. Clinical evaluation of animal diabetes (cats and dogs) include hyperglycemia, physical exam, complete blood count [CBC], Elevated blood glucose (BG), glucosuria, chemistry with electrolytes, urine analysis with culture, urine protein creatinine ratio (UPC), triglycerides, blood pressure (BP), and thyroxine (T4). While the level of BG concentration elevated to 200 mg/dL in dogs and 250–300 mg/dL in cats, glucosuria will typically occur. Pets with persistent glucosuria, persistent hyperglycemia, and presence clinical signs would be judged as diabetes mellitus (DM). Furthermore, HbA1c now have become a crucial glycemic indicator for diabetic dogs. Neslihan Tascene et al. revealed that blood HbA1c levels of diabetic were found to be 3.11±0.4 %, whereas the normal dog was 1.07±0.08 % respectively. Besides, the blood serum glucose level of diabetic dogs was around 526.71±22 mg/dl, whereas blood sugar in control dogs were around 97.80±2.93 mg/dl. Hasegawa S. demonstrated 6.41% HbA1c in diabetic dogs, whereas normal dogs with 2.6% (mean HbA1c of total Hb, %). And mean HbA1 values of normal dogs and diabetic dogs were 3.58 and 7.41%. In addition, Na-Yon Kim et al. showed significantly higher HbA1c concentrations of diabetic dogs (>6.2%) than non-diabetic dogs (p < 0.001) with commercial HbA1c testing system. Furthermore, Chao-Nan Lin et al. present the stability of canine glycosylated hemoglobin sample at room (25°C) and refrigerator (4°C) temperatures over 14 days. Besides, different purified methods would cause slight variation in measuring HbA1c value. Monitoring of pets’ diabetes Monitoring options include performance of blood glucose curves (BGCs), monitoring urine glucose (UG), measuring fructosamine, and assessment of clinical signs and weight. a. Blood glucose (BG) levels: Blood glucose levels fluctuate and could be used for indicating short periods of hyperglycemia. Normal BG were 63~110mg/dL in dogs; 47~151mg/dL in cats. As we mentioned, when the BG concentration goes over approximately 200 mg/dL in dogs and 250–300 mg/dL in cats, Glucosuria will occur. Blood Glucose Curves should be established during insulin treatment. b. Threshold of urine glucose (UG): UG concentration reflects only the average BG. Thus, it’s not recommended to solely rely on UG measurements is not recommended. Regardless, UG concentration can assist in assessment of DM together with other evaluation parameters. The threshold of urine glucose (UG) would fall in 180mg/dL in dogs; 252mg/dL in cats. c. Fructosamine: Fructosamine is a glycosylated protein formed by nonenzymatic, irreversible binding of glucose to serum albumin. It able to discern normal glycemic level from diabetes with chronic hyperglycemia and won’t be affected by
Case study: Feline infectious peritonitis in a cat presented because of papular skin lesions
Case study: Feline infectious peritonitis in a cat presented because of papular skin lesions Robert Lo, Ph.D, D.V.M A 19-week-old neutered male domestic shorthair cat presented only multiple raised pruritic skin lesions along the dorsal head and back and no other symptoms. The cat showed poor appetite and spreading of the skin lesions five days after initial treatment, and then biopsies were taken and submitted to the dermatopathology service. Histopathology indicated strong suspicion of FIP. With cat’s health getting worse, euthanasia was performed. Then, necropsy was also performed. Abundant viscous serofibrinous effusions were found in the abdomen, thorax, and pericardium. Multiple white nodules were observed in the lungs, liver, and kidney. Histologic examination revealed multifocal to coalescing areas of pyogranulomatous inflammation in the affected tissues. Dermal necrosis was observed in skin sections. Immunohistochemical staining for intracellular feline coronavirus showed positive staining within the cytoplasm of macrophages in the lung, kidney, skin, and brain. Feline infectious peritonitis with associated cutaneous lesions was diagnosed in this cat based on gross and histologic lesions along with immunohistochemistry results. Figure 1: A — Skin lesions (multiple round raised skin nodules) on the dorsal surface of the head and neck. B —Dermal necrosis was observed in histological sections of skin; H&E. C —Immunohistochemical staining for intracellular feline coronavirus showed positive brown staining in skin section. (Redford T, Al-Dissi AN. Feline infectious peritonitis in a cat presented because of papular skin lesions., Can Vet J. 2019 Feb;60(2):183-185.) Original paper: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6340254/
Breed-related disease: Bulldogs

The Bulldog also known as the English bulldog is a medium-sized dog breed, descended from dogs originally used for blood sports in the 16th century, at that time, the bulldog was bred with the purpose of bull-baiting in mind, which required the animal to be both strong and tenacious with vicious fighting spirit. Today, however, the modern English bulldog is worlds apart from the fighting dog it was originally. The English bulldog we all know today has been selectively bred over a few hundred years to serve as a companion animal. Although to some the Bulldog can appear intimidating, the breed has developed a great reputation for gentle play with children and tolerance for other household pets. The bulldog is an iconic breed for its wide-set frame, muscular physic, and compact stubby legs. The body is stocky and dense and the head is large and wide. A hallmark of the breed is the folds of skin that form around the face and forehead of the animal, together with the drooping cheeks that extend from each eye. Their loyal and steadfast determination remains from their bull-baiting days, however, and good training from a young age, paired with firm and consistent discipline is a must when owning an English bulldog. They are predominantly housed bound pets and require a good walk at least once a day. Unfortunately, the Bulldog’s unique body and head structure make him prone to several health problems, here are some of the most common diseases related to the bulldogs: Brachycephalic Airway Syndrome _ Brachycephalic is Latin for smooched face, and every English bulldog has Brachycephalic Airway Syndrome (BAS) to some degree. BAS is common in animals that have shortened facial features that give them the pushed-in nose. According to the ACVS, bulldogs “have been bred to have relatively short muzzles and noses and, because of this, the throat and breathing passages in these dogs are frequently undersized or flattened.” Their noses are narrow, and the bones on their face are shorter, which causes an array of health risks: Breathing problems and panting chronic discomfort, Exercise intolerance, Difficulty eating. Difficulty breathing _ when looking at the root cause of health risks in bulldogs, upper airway defects caused by Brachycephalic Airway Syndrome wreak a lot of havoc. No one wants their pooch to struggle to take a breath, but unfortunately, breathing can be a big problem for bulldogs. Genetic abnormalities caused by selective breeding have a significant impact on your pup’s airways. Skin Problems _ sadly, the adorable folds bulldog owners know and love have a downside. Some English bulldog health problems symptoms include skin infections and irritation. A. Eczema, or “canine atopic dermatitis,” is the most common skin issue found in bulldogs. It causes itchy, dried-out skin that can turn into a scaly rash. Allergies, stress, and bug bites are the most common causes. B. Bacterial infections like staph, pyoderma, and dermatitis also can occur. These infections can either be surface level or go deeper underneath the skin. c. Hot spots, or “acute moist dermatitis,” are an allergic reaction to different skin irritants like bug bites and parasites and appear as round sores on the skin. English Bulldogs also can suffer from acne caused by dirty pores. d. Interdigital cysts are also common in bulldogs. Cysts form between the toes, swelling into large bumps. Treat cysts with a simple cleaning, but be careful not to overdo it. Excessive cleaning can worsen the condition. Thyroid and Heart Disease Health issues from selective breeding have also caused problems in the internal organs. Thyroid – Hyperthyroidism is when the thyroid slows down, which causes decreased production of thyroxine, the hormone responsible for regulating the metabolism. Heart – Pulmonary Stenosis which is a heart deformity most often found in English Bulldogs. According to UFAW, Pulmonary Stenosis is a “congenital narrowness or constriction of the outflow from the right side of the heart.” It blocks blood flow and can lead to heart failure or even death. You can catch this disease early with regular heart assessments at checkups. Cancer _ Bulldogs are especially susceptible to cancers like lymphoma and mast cell tumors. https://en.wikipedia.org/wiki/Bulldog https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0217928 https://www.lucypetproducts.com/blog/10-common-english-bulldog-health-issues/ https://yourdogadvisor.com/bulldog-mix/ Photo credit: https://chantelbulldogs.com/product/english-bulldog-puppies-for-sale/ https://www.pinterest.com.au/pin/685110162039360718/ https://unsplash.com/s/photos/english-bulldog
Breed-related disease: Maine Coon

John K. Rosembert The Maine Coon is the largest domesticated cat breed, it has a distinctive physical appearance and valuable hunting skills, the Maine Coon is solid, rugged, and can endure a harsh climate. Maine Coons, like American Shorthairs, are considered native to America because they ‘ve been on this continent since the colonial days, and perhaps longer. How they got here in the first place and where their progenitors came from, however, is anyone’s guess, since no records of the Maine Coon’s exact origins and date of introduction to the America exist, so several competing hypotheses have been suggested, the most credible suggestion being that it is closely related to the Norwegian Forest cat and the Siberian. The Maine Coon is a big, rugged cat with a smooth, shaggy coat with a well-proportioned body that is muscular and broad-chested. It has substantial, medium-length legs and large, round paws, well tufted with fur, to serve as “snowshoes” during winter. A heavy coat is shorter on the shoulders, longer on the stomach and britches (long fur on the upper hind legs), with a ruff in front and a long, furry tail waving a greeting. A medium-width head is slightly longer than it is wide and has a squarish muzzle. Large, well-tufted ears are wide at the base, tapering to a point, and large, expressive eyes are green, gold, greenish-gold or copper. White or bi-colored Maine Coons may have blue or odd eyes. The friendly, laid back Maine Coon is a perfect choice for families with children and cat-friendly dogs. They love the attention they receive from children who treat them politely and with respect, and they don’t mind playing dress -up or going for a ride in a baby buggy. They’re happy to live with cat-friendly dogs, too, thanks to their amiable disposition. Introduce pets slowly and in controlled circumstances to ensure that they learn to get along together. While Maine coon cats are generally healthy, some inherit genetic diseases that can shorten life, cause pain or decrease mobility. Here listed below some of the most common Maine coon related diseases. 1. Hip Dysplasia: which Maine Coons and Persians are said to be more prone to, is the failure of the hip joints to develop correctly. This leads to a deteriorating hip joint and eventually a complete loss of function. Both Maine Coons and Persians are both ‘heavy boned’ cats. 2. Spinal Muscular Atrophy (SMA): This is a degenerative disorder affecting the spinal cord to the rear legs. An affected kitten will lose its trademark steadiness and cat like abilities. 3. Hypertrophic Cardiomyopathy (HCM): Hypertrophic Cardiomyopathy (HCM) is essentially a thickening of the heart tissue with scar tissue. It’s very tricky to diagnose, and is often called a ‘silent killer’. More middle aged and older Maine Coons are predisposed to it, whereby the heart becomes too muscular. This leads to a distortion within the heart muscle where the left ventricle becomes smaller, leading to abnormal heart rates. 4. Polycystic Kidney Disease (PKD): In a disease that is said to affect up to 6% of cats annually, you may well be in good company if you find your Maine Coon has this condition. PKD has a wide scope of disorder. Many Maine Coons can lead happy and fulfilling lives before succumbing to something else, whereas others, with a fatal level of PKD will succumb earlier to chronic renal failure. Sources: https://www.petfinder.com/cat-breeds/maine-coon/ https://www.harlingenveterinaryclinic.com/services/cats/breeds/maine-coon https://cattime.com/cat-breeds / maine-coon-cats # / slide / 1 https://mainecoonexpert.com/what-are-the-common-maine-coon-health-problems-full-guide/ Photo credit: https://catzinc.org/ breeders-list / maine-coon /
Breed-related disease: Pomeranian

John K.Rosembert The Pomeranian (often known as a Pom) Although the Pomeranian (also called Zwergspitz, Dwarf Spitz, Loulou) is a breed of dog of the Spitz type that is named for the Pomerania region in north-west Poland and north-east Germany in Central Europe. They only weighs from three to seven pounds, The Pomeranian is the smallest member of the Spitz family of dogs, which includes the Samoyed, Alaskan malamute, and Norwegian elkhound, among others. Poms take their name from the province of Pomerania, in Germany. They became especially popular when Queen Victoria allowed some of her Pomeranians to be shown in a conformation show, the first Pomeranians ever to be shown. Cute, feisty and furry, Poms are intelligent and loyal to their families. Don’t let their cuteness fool you, however. These independent, bold dogs have minds of their own. They are alert and curious about the world around them. Unfortunately, in their minds, they are much larger than they really are, which can sometimes lead them to harass and even attack much larger dogs. Luckily, if they are properly socialized with other dogs and animals, they generally get along quite well with them. Here are some of the most common diseases related to the breed Pomeranian: 1. Luxating patellas _ (knees that slip out of place) are the most common problem in the Pomeranian breed. The knees are graded according to the OFA (Orthopedic Foundation for Animals). 2. Hypothyroidism _ (low thyroid) is very common in the Pomeranian breed. Health testing for a normal thyroid is an “optional” test recommended. 3. Collapsed Trachea Pomeranians who make honking noises or cough-like sounds (much like a cat regurgitating a hairball) may have collapsed tracheas. An x-ray can diagnose the issue. 4. Hair Loss _ this problem is often referred to as Black skin disease, BSD, or Alopecia X. An accurate diagnosis is often a very long, inconclusive and expensive exercise. Many Pomeranian skin conditions can be the cause of the problem, such as Hypothyroidism or low thyroid, Cushing’s disease, eczema, mites, fungus infections and allergies. 5. Hypoglycemia _ can occur in young Pomeranians, It is more common in the very small or very active puppies. Be sure that your breeder gives you complete instructions on how to determine if your puppy is starting to develop hypoglycemia. It is a problem that the puppy outgrows as they mature. Adult hypoglycemia is a serious metabolic disorder. Dogs who have this should not be bred. 6. Seizures or idiopathic epilepsy _ known as idiopathic because the cause is not known and epilepsy basically means repeat seizures. Seizures might happen as an onetime occurrence for numerous reasons, however, if the seizures are repetitive this is called epilepsy. Sources: https://dogtime.com/dog-breeds/pomeranian#/slide/1 https://www.dogzhealth.com/pomeranian-health-problems/ http://cdn.akc.org/Marketplace/Health-Statement/Pomeranian.pdf Photo credit: https://pixabay.com/photos/dog-pomeranian-cute-animal-canine-1113398/
Feline Immunodeficiency Virus (FIV): Virology, Diagnosis & Management
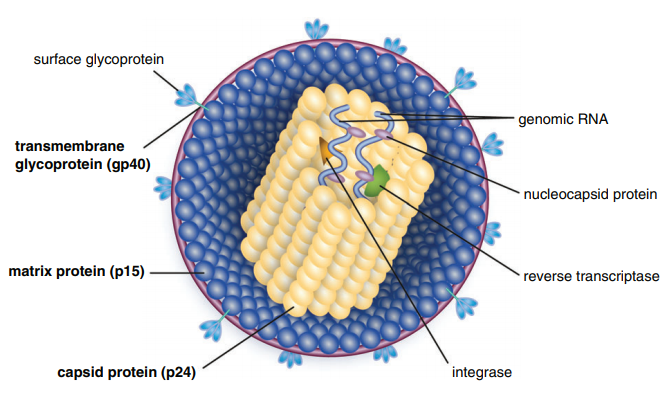
Table of Contents Maigan Espinili Maruquin 1. Introduction: The Scientific and Clinical Significance of FIV 1.1 Historical Discovery of FIV (1986) and Its Relevance as an HIV Model Feline Immunodeficiency Virus (FIV) was first recognized in 1986, when domestic cats in California presented with severe immunodeficiency syndromes remarkably similar to human AIDS. In 1987, Pedersen, Ho, and colleagues successfully isolated the virus, identifying it as a novel feline lentivirus and establishing the foundation for a new model of lentiviral immunopathogenesis (Pedersen, Ho et al. 1987). Initial observations emphasized that affected cats showed clinical signs of profound immune dysfunction—fever, lymphadenopathy, recurrent infections—but were negative for Feline Leukemia Virus (FeLV), differentiating this syndrome from previously known retroviral diseases in cats (Harbour, D.A. et al. 2004). Because FIV belongs to the Lentivirus genus within Retroviridae, similar to Human Immunodeficiency Virus (HIV), it was subsequently named in parallel with HIV. The biological, genomic, and pathological similarities between FIV and HIV—including their shared tropism for CD4⁺ T lymphocytes, slow progression, and lifelong persistence—positioned FIV early on as an important natural animal model for studying human AIDS (Elder & Phillips 1995; Miller, Cairns et al. 2000; Troyer, Pecon-Slattery et al. 2005; Hosie, Techakriengkrai et al. 2017). To this day, FIV remains a critical comparative model for HIV vaccine development, antiviral therapy research, and studies of lentiviral immune dysfunction. 1.2 Classification as a Lentivirus and Early Pathogenesis Studies FIV is a member of the Lentivirus genus, characterized by chronic, progressive infections and slow viral replication cycles. Like HIV, FIV integrates its genome into the host’s DNA to form a provirus, resulting in lifelong infection (Bendinelli, Pistello et al. 1995; Westman, Malik et al. 2019). Three Major Clinical Stages of Infection FIV infection progresses through a series of well-defined clinical stages that mirror HIV pathogenesis: Acute (Primary) Infection Occurs 1–6 weeks post-exposure and may last 1–4 weeks. Common findings include:• transient fever, lethargy, dullness• generalized lymphadenopathy• transient neutropenia• anorexia, diarrhea• mild upper respiratory signs(Ishida & Tomoda 1990; Bendinelli, Pistello et al. 1995) During this phase, the thymus and lymphoid tissues are important early replication sites where lesions may appear within 4 weeks (Harbour, D.A. et al. 2004). Asymptomatic (Latent) Phase Often lasts 1.5–2 years or longer.• Cats appear healthy but remain persistently infected.• Viral replication continues at low levels.• Subclinical chronic inflammatory lesions may occur (Harbour, D.A. et al. 2004). Terminal Immunodeficiency Phase (Feline AIDS; FAIDS) Characterized by:• severe immune collapse• high viral replication• chronic secondary infections• neoplasia (especially lymphomas)• neurological abnormalities(Pedersen 1993; Bendinelli, Pistello et al. 1995; Westman, Malik et al. 2019) These stages reflect lentiviral dynamics nearly identical to HIV progression in humans. 1.3 Distinction Between FIV and FeLV in Naturally Infected Cats Although both FIV and FeLV are important feline retroviruses, they differ significantly in classification, transmission, and pathogenesis: Classification and Molecular Differences FIV: Lentivirus; elongated morphology; Mg²⁺-dependent reverse transcriptase (Harbour, D.A. et al. 2004). FeLV: Gammaretrovirus; Mn²⁺-dependent reverse transcriptase. Transmission FIV: Primarily transmitted via bite wounds during fighting or mating aggression (Perharic, Bidin et al. 2016; Miller, Boegler et al. 2017). FeLV: Transmitted via casual contact: grooming, shared bowls, and vertical passage. Clinical Outcome FIV:• Lifelong persistent infection• Slow immunodeficiency progression• Many cats live normal or near-normal lifespans with appropriate care FeLV:• More acutely pathogenic• Causes immunosuppression, anemia, lymphoma• Can manifest as abortive, regressive, or progressive infection These differences require distinct diagnostic and management approaches. 1.4 Why FIV Remains a Critical Feline Pathogen in Modern Veterinary Medicine Global Prevalence and Risk Factors FIV prevalence varies with geography, lifestyle, and demographics:• 2.5–5 percent among healthy cats in North America• Up to ≥15 percent in high-risk populations (Bendinelli, Pistello et al. 1995)• Higher in older, free-roaming, intact male cats due to aggressive behaviors Pathogenesis: Progressive Immune Dysfunction FIV induces a chronic decline in immune function via:• depletion of CD4⁺ T cells• inversion of the CD4:CD8 ratio• chronic immune activation• susceptibility to secondary infections These immune abnormalities closely mirror HIV-induce immunopathology. Neoplastic and Neurological Sequelae FIV markedly increases the risk of neoplasia, especially B-cell lymphosarcoma.Neurological manifestations include:• seizures• altered behavior• dementia• ataxia(Ishida & Tomoda 1990; Bendinelli, Pistello et al. 1995) Transmission Challenges Although FIV is shed in saliva, deep bite wounds remain the dominant transmission route. Viral RNA, DNA, and antibodies have been detected in saliva and oral tissues (Yamamoto, Sparger et al. 1988; Pedersen, Yamamoto et al. 1989; Miller, Boegler et al. 2017). Ethical and Clinical Implications Because many FIV-positive cats remain healthy for years:• A positive test result must never be the sole basis for euthanasia (Harbour, D.A. et al. 2004).• Appropriate counseling is required to prevent mismanagement.• Early diagnosis enables interventions that improve longevity and quality of life. Importance in HIV Research The strong biological parallels between FIV and HIV continue to position FIV as a unique model for:• immune dysfunction• neurological disease• vaccine development• antiretroviral therapy testing(Hosie, Techakriengkrai et al. 2017) 2. Virology and Molecular Biology 2.1 Viral Structure Feline Immunodeficiency Virus (FIV) is a member of the Lentivirus genus within the Retroviridae family. Mature virions measure approximately 100–110 nm in diameter and contain a characteristic cylindrical nucleocapsid core. The virion is enclosed by a host-derived lipid bilayer acquired during budding from the plasma membrane of infected cells. Embedded within this lipid envelope are short surface glycoprotein spikes that are fundamental to viral infectivity and immune recognition. The viral Env glycoprotein precursor undergoes proteolytic cleavage to produce two mature subunits: Surface unit (SU, gp95) Transmembrane unit (TM, gp40) These subunits are non-covalently associated in a trimeric structure within the viral membrane. Together, they mediate: Receptor recognition and binding Membrane fusion and viral entry Immune evasion and antigenicity, serving as major targets for neutralizing antibodies The gp40 transmembrane glycoprotein is a key antigen detected by many point-of-care diagnostic kits, underscoring its immunological relevance. Within the core, the virion contains two identical positive-sense RNA strands assembled with nucleocapsid proteins, essential viral enzymes, and structural components. This genome–nucleoprotein complex is enclosed by the capsid protein (CA, p24), itself surrounded by the matrix protein (MA, p14)
