Canine HbA1c
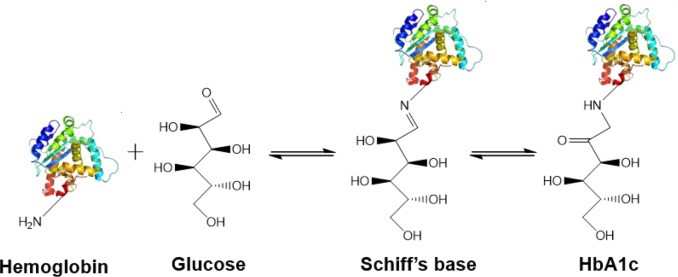
Canine HbA1c LIN, WEN-YANG (WESLEY), Ph.D HbA1c is a type of hemoglobin on which several monosaccharides such as glucose, galactose and fructose tend to bind with and exist in the bloodstream. The chemical linking process between sugar and hemoglobin is named glycation. HbA1c is actually an indicator of the beta-N-1-deoxy fructosyl on hemoglobin, which has been used as diagnostic measurement of long-term glycemic control for patients with diabetes mellitus. The increasing number of HbA1c in bloodstream represent the elevated plasma glucose level that usually indicating a poor diabetic management may lead to severer conditions. Due to the lifecycle of red blood cells is average four months, the HbA1c test could effectively present the real blood sugar degree in latest three months. In 1980, Wood and Smith first demonstrated the applicable method of examining canine diabetes with monitoring glycosylated haemoglobin. Later, Smith et al 1982, Mahaffey and Cornelius 1982, Dennis 1989, Jensen 1995 all confirmed this method is workable on diagnosing diabetic dogs. Molecular structure of HbA1c Figure 1 showed an aldimine linkage between glucose and hemoglobin (Figure1). Figure 1. Glycation process between Hemoglobin and glucose https://www.sciencedirect.com/science/article/pii/S0956566318304500 As we previously discussed that different monosaccharide would form different chemical structure with blood cells. Thus, while using cation exchange chromatography to separate Hemoglobin type A, different fractions would be separate out like HbA0, HbA1a, HbA1a2, HbA1b, and HbA1c. Fractions were named after eluting order (Figure 2). Figure 2. Different separated fractions of Hemoglobin type A with cation exchange chromatography https://www.sciencedirect.com/science/article/abs/pii/S0003269711000753 Glycated hemoglobin forming harmful factors in human body Highly reactive free radicals promote the formation of abnormal ferryl Hb (Fe4+-Hb), which enhance macrophage accumulation in blood vessel. Both macrophage accumulation and accumulation of glycated hemoglobin would elevate blood viscosity and slow down normal blood flow. Thus, atherosclerotic plaque would gradually occur in blood vessel. History of standardized HbA1c as diagnostic tool for diabetes Diabetes has become a serious health issue in entire world, due to over 220 million patients are suffering of it. Among all cases of diabetes, type 2 diabetes take majority part (90% to 95%). Besides, worsen type 2 diabetes could also cause extra complications, such as cardiovascular diseases, peripheral neuropathy, nephropathy, optic neuropathy, diabetic foot and even unto death. Several risks factor related to diabetes including high fat diet, obesity, smoking, elevated cholesterol levels, high blood pressure and lack of regular exercise. For preventing and relieving conditions of diabetes, taking healthy diet and regular exercise are important. In addition, diabetic patients’ blood sugar condition should be monitored regularly all the time. There are many clinical methods to evaluate glycemia like urine glucose, random or fasting plasma glucose etc. However, HbA1c is considered to be one of the most accurate and efficient method for measuring long-term blood sugar level (3 to 4 months). From 1894 to 1993, Diabetes Control and Complications Trial (DCCT) established the big data of diabetic patients’ HbA1c values to mean blood glucose resulted in making HbA1c a reliable index of mean blood glucose. However, DCCT haven’t standardized the HbA1c assay methods for labs and clinics. Later, the American Association for Clinical Chemistry (AACC) Standards Committee established a HbA1c Standardization Subcommittee to develop a plan for standardizing HbA1c assay that clinical laboratories could take advantage of it to perform precise glycemic evaluation and control. Now, the National Glycohemoglobin Standardization Program (NGSP) continue to develop reliable assays of HbA1c. Diagnosing canine diabetes with HbA1c 2018 American Animal Hospital Association suggested guidelines of diagnosis and assessment for animal diabetes. Clinical evaluation of animal diabetes (cats and dogs) include hyperglycemia, physical exam, complete blood count [CBC], Elevated blood glucose (BG), glucosuria, chemistry with electrolytes, urine analysis with culture, urine protein creatinine ratio (UPC), triglycerides, blood pressure (BP), and thyroxine (T4). While the level of BG concentration elevated to 200 mg/dL in dogs and 250–300 mg/dL in cats, glucosuria will typically occur. Pets with persistent glucosuria, persistent hyperglycemia, and presence clinical signs would be judged as diabetes mellitus (DM). Furthermore, HbA1c now have become a crucial glycemic indicator for diabetic dogs. Neslihan Tascene et al. revealed that blood HbA1c levels of diabetic were found to be 3.11±0.4 %, whereas the normal dog was 1.07±0.08 % respectively. Besides, the blood serum glucose level of diabetic dogs was around 526.71±22 mg/dl, whereas blood sugar in control dogs were around 97.80±2.93 mg/dl. Hasegawa S. demonstrated 6.41% HbA1c in diabetic dogs, whereas normal dogs with 2.6% (mean HbA1c of total Hb, %). And mean HbA1 values of normal dogs and diabetic dogs were 3.58 and 7.41%. In addition, Na-Yon Kim et al. showed significantly higher HbA1c concentrations of diabetic dogs (>6.2%) than non-diabetic dogs (p < 0.001) with commercial HbA1c testing system. Furthermore, Chao-Nan Lin et al. present the stability of canine glycosylated hemoglobin sample at room (25°C) and refrigerator (4°C) temperatures over 14 days. Besides, different purified methods would cause slight variation in measuring HbA1c value. Monitoring of pets’ diabetes Monitoring options include performance of blood glucose curves (BGCs), monitoring urine glucose (UG), measuring fructosamine, and assessment of clinical signs and weight. a. Blood glucose (BG) levels: Blood glucose levels fluctuate and could be used for indicating short periods of hyperglycemia. Normal BG were 63~110mg/dL in dogs; 47~151mg/dL in cats. As we mentioned, when the BG concentration goes over approximately 200 mg/dL in dogs and 250–300 mg/dL in cats, Glucosuria will occur. Blood Glucose Curves should be established during insulin treatment. b. Threshold of urine glucose (UG): UG concentration reflects only the average BG. Thus, it’s not recommended to solely rely on UG measurements is not recommended. Regardless, UG concentration can assist in assessment of DM together with other evaluation parameters. The threshold of urine glucose (UG) would fall in 180mg/dL in dogs; 252mg/dL in cats. c. Fructosamine: Fructosamine is a glycosylated protein formed by nonenzymatic, irreversible binding of glucose to serum albumin. It able to discern normal glycemic level from diabetes with chronic hyperglycemia and won’t be affected by
Case study: Feline infectious peritonitis in a cat presented because of papular skin lesions
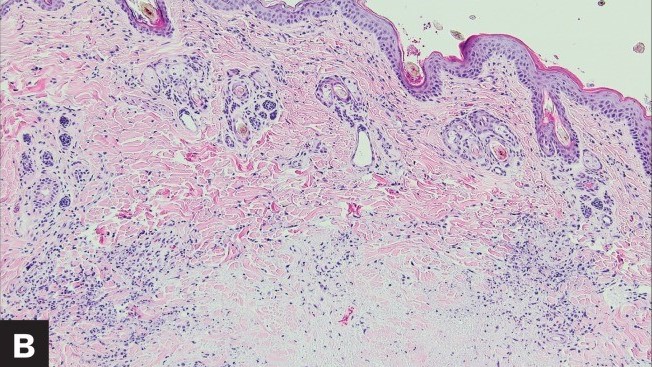
Case study: Feline infectious peritonitis in a cat presented because of papular skin lesions Robert Lo, Ph.D, D.V.M A 19-week-old neutered male domestic shorthair cat presented only multiple raised pruritic skin lesions along the dorsal head and back and no other symptoms. The cat showed poor appetite and spreading of the skin lesions five days after initial treatment, and then biopsies were taken and submitted to the dermatopathology service. Histopathology indicated strong suspicion of FIP. With cat’s health getting worse, euthanasia was performed. Then, necropsy was also performed. Abundant viscous serofibrinous effusions were found in the abdomen, thorax, and pericardium. Multiple white nodules were observed in the lungs, liver, and kidney. Histologic examination revealed multifocal to coalescing areas of pyogranulomatous inflammation in the affected tissues. Dermal necrosis was observed in skin sections. Immunohistochemical staining for intracellular feline coronavirus showed positive staining within the cytoplasm of macrophages in the lung, kidney, skin, and brain. Feline infectious peritonitis with associated cutaneous lesions was diagnosed in this cat based on gross and histologic lesions along with immunohistochemistry results. Figure 1: A — Skin lesions (multiple round raised skin nodules) on the dorsal surface of the head and neck. B —Dermal necrosis was observed in histological sections of skin; H&E. C —Immunohistochemical staining for intracellular feline coronavirus showed positive brown staining in skin section. (Redford T, Al-Dissi AN. Feline infectious peritonitis in a cat presented because of papular skin lesions., Can Vet J. 2019 Feb;60(2):183-185.) Original paper: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6340254/
Breed-related disease: Bulldogs

The Bulldog also known as the English bulldog is a medium-sized dog breed, descended from dogs originally used for blood sports in the 16th century, at that time, the bulldog was bred with the purpose of bull-baiting in mind, which required the animal to be both strong and tenacious with vicious fighting spirit. Today, however, the modern English bulldog is worlds apart from the fighting dog it was originally. The English bulldog we all know today has been selectively bred over a few hundred years to serve as a companion animal. Although to some the Bulldog can appear intimidating, the breed has developed a great reputation for gentle play with children and tolerance for other household pets. The bulldog is an iconic breed for its wide-set frame, muscular physic, and compact stubby legs. The body is stocky and dense and the head is large and wide. A hallmark of the breed is the folds of skin that form around the face and forehead of the animal, together with the drooping cheeks that extend from each eye. Their loyal and steadfast determination remains from their bull-baiting days, however, and good training from a young age, paired with firm and consistent discipline is a must when owning an English bulldog. They are predominantly housed bound pets and require a good walk at least once a day. Unfortunately, the Bulldog’s unique body and head structure make him prone to several health problems, here are some of the most common diseases related to the bulldogs: Brachycephalic Airway Syndrome _ Brachycephalic is Latin for smooched face, and every English bulldog has Brachycephalic Airway Syndrome (BAS) to some degree. BAS is common in animals that have shortened facial features that give them the pushed-in nose. According to the ACVS, bulldogs “have been bred to have relatively short muzzles and noses and, because of this, the throat and breathing passages in these dogs are frequently undersized or flattened.” Their noses are narrow, and the bones on their face are shorter, which causes an array of health risks: Breathing problems and panting chronic discomfort, Exercise intolerance, Difficulty eating. Difficulty breathing _ when looking at the root cause of health risks in bulldogs, upper airway defects caused by Brachycephalic Airway Syndrome wreak a lot of havoc. No one wants their pooch to struggle to take a breath, but unfortunately, breathing can be a big problem for bulldogs. Genetic abnormalities caused by selective breeding have a significant impact on your pup’s airways. Skin Problems _ sadly, the adorable folds bulldog owners know and love have a downside. Some English bulldog health problems symptoms include skin infections and irritation. A. Eczema, or “canine atopic dermatitis,” is the most common skin issue found in bulldogs. It causes itchy, dried-out skin that can turn into a scaly rash. Allergies, stress, and bug bites are the most common causes. B. Bacterial infections like staph, pyoderma, and dermatitis also can occur. These infections can either be surface level or go deeper underneath the skin. c. Hot spots, or “acute moist dermatitis,” are an allergic reaction to different skin irritants like bug bites and parasites and appear as round sores on the skin. English Bulldogs also can suffer from acne caused by dirty pores. d. Interdigital cysts are also common in bulldogs. Cysts form between the toes, swelling into large bumps. Treat cysts with a simple cleaning, but be careful not to overdo it. Excessive cleaning can worsen the condition. Thyroid and Heart Disease Health issues from selective breeding have also caused problems in the internal organs. Thyroid – Hyperthyroidism is when the thyroid slows down, which causes decreased production of thyroxine, the hormone responsible for regulating the metabolism. Heart – Pulmonary Stenosis which is a heart deformity most often found in English Bulldogs. According to UFAW, Pulmonary Stenosis is a “congenital narrowness or constriction of the outflow from the right side of the heart.” It blocks blood flow and can lead to heart failure or even death. You can catch this disease early with regular heart assessments at checkups. Cancer _ Bulldogs are especially susceptible to cancers like lymphoma and mast cell tumors. https://en.wikipedia.org/wiki/Bulldog https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0217928 https://www.lucypetproducts.com/blog/10-common-english-bulldog-health-issues/ https://yourdogadvisor.com/bulldog-mix/ Photo credit: https://chantelbulldogs.com/product/english-bulldog-puppies-for-sale/ https://www.pinterest.com.au/pin/685110162039360718/ https://unsplash.com/s/photos/english-bulldog
Breed-related disease: Maine Coon

John K. Rosembert The Maine Coon is the largest domesticated cat breed, it has a distinctive physical appearance and valuable hunting skills, the Maine Coon is solid, rugged, and can endure a harsh climate. Maine Coons, like American Shorthairs, are considered native to America because they ‘ve been on this continent since the colonial days, and perhaps longer. How they got here in the first place and where their progenitors came from, however, is anyone’s guess, since no records of the Maine Coon’s exact origins and date of introduction to the America exist, so several competing hypotheses have been suggested, the most credible suggestion being that it is closely related to the Norwegian Forest cat and the Siberian. The Maine Coon is a big, rugged cat with a smooth, shaggy coat with a well-proportioned body that is muscular and broad-chested. It has substantial, medium-length legs and large, round paws, well tufted with fur, to serve as “snowshoes” during winter. A heavy coat is shorter on the shoulders, longer on the stomach and britches (long fur on the upper hind legs), with a ruff in front and a long, furry tail waving a greeting. A medium-width head is slightly longer than it is wide and has a squarish muzzle. Large, well-tufted ears are wide at the base, tapering to a point, and large, expressive eyes are green, gold, greenish-gold or copper. White or bi-colored Maine Coons may have blue or odd eyes. The friendly, laid back Maine Coon is a perfect choice for families with children and cat-friendly dogs. They love the attention they receive from children who treat them politely and with respect, and they don’t mind playing dress -up or going for a ride in a baby buggy. They’re happy to live with cat-friendly dogs, too, thanks to their amiable disposition. Introduce pets slowly and in controlled circumstances to ensure that they learn to get along together. While Maine coon cats are generally healthy, some inherit genetic diseases that can shorten life, cause pain or decrease mobility. Here listed below some of the most common Maine coon related diseases. 1. Hip Dysplasia: which Maine Coons and Persians are said to be more prone to, is the failure of the hip joints to develop correctly. This leads to a deteriorating hip joint and eventually a complete loss of function. Both Maine Coons and Persians are both ‘heavy boned’ cats. 2. Spinal Muscular Atrophy (SMA): This is a degenerative disorder affecting the spinal cord to the rear legs. An affected kitten will lose its trademark steadiness and cat like abilities. 3. Hypertrophic Cardiomyopathy (HCM): Hypertrophic Cardiomyopathy (HCM) is essentially a thickening of the heart tissue with scar tissue. It’s very tricky to diagnose, and is often called a ‘silent killer’. More middle aged and older Maine Coons are predisposed to it, whereby the heart becomes too muscular. This leads to a distortion within the heart muscle where the left ventricle becomes smaller, leading to abnormal heart rates. 4. Polycystic Kidney Disease (PKD): In a disease that is said to affect up to 6% of cats annually, you may well be in good company if you find your Maine Coon has this condition. PKD has a wide scope of disorder. Many Maine Coons can lead happy and fulfilling lives before succumbing to something else, whereas others, with a fatal level of PKD will succumb earlier to chronic renal failure. Sources: https://www.petfinder.com/cat-breeds/maine-coon/ https://www.harlingenveterinaryclinic.com/services/cats/breeds/maine-coon https://cattime.com/cat-breeds / maine-coon-cats # / slide / 1 https://mainecoonexpert.com/what-are-the-common-maine-coon-health-problems-full-guide/ Photo credit: https://catzinc.org/ breeders-list / maine-coon /
Breed-related disease: Pomeranian

John K.Rosembert The Pomeranian (often known as a Pom) Although the Pomeranian (also called Zwergspitz, Dwarf Spitz, Loulou) is a breed of dog of the Spitz type that is named for the Pomerania region in north-west Poland and north-east Germany in Central Europe. They only weighs from three to seven pounds, The Pomeranian is the smallest member of the Spitz family of dogs, which includes the Samoyed, Alaskan malamute, and Norwegian elkhound, among others. Poms take their name from the province of Pomerania, in Germany. They became especially popular when Queen Victoria allowed some of her Pomeranians to be shown in a conformation show, the first Pomeranians ever to be shown. Cute, feisty and furry, Poms are intelligent and loyal to their families. Don’t let their cuteness fool you, however. These independent, bold dogs have minds of their own. They are alert and curious about the world around them. Unfortunately, in their minds, they are much larger than they really are, which can sometimes lead them to harass and even attack much larger dogs. Luckily, if they are properly socialized with other dogs and animals, they generally get along quite well with them. Here are some of the most common diseases related to the breed Pomeranian: 1. Luxating patellas _ (knees that slip out of place) are the most common problem in the Pomeranian breed. The knees are graded according to the OFA (Orthopedic Foundation for Animals). 2. Hypothyroidism _ (low thyroid) is very common in the Pomeranian breed. Health testing for a normal thyroid is an “optional” test recommended. 3. Collapsed Trachea Pomeranians who make honking noises or cough-like sounds (much like a cat regurgitating a hairball) may have collapsed tracheas. An x-ray can diagnose the issue. 4. Hair Loss _ this problem is often referred to as Black skin disease, BSD, or Alopecia X. An accurate diagnosis is often a very long, inconclusive and expensive exercise. Many Pomeranian skin conditions can be the cause of the problem, such as Hypothyroidism or low thyroid, Cushing’s disease, eczema, mites, fungus infections and allergies. 5. Hypoglycemia _ can occur in young Pomeranians, It is more common in the very small or very active puppies. Be sure that your breeder gives you complete instructions on how to determine if your puppy is starting to develop hypoglycemia. It is a problem that the puppy outgrows as they mature. Adult hypoglycemia is a serious metabolic disorder. Dogs who have this should not be bred. 6. Seizures or idiopathic epilepsy _ known as idiopathic because the cause is not known and epilepsy basically means repeat seizures. Seizures might happen as an onetime occurrence for numerous reasons, however, if the seizures are repetitive this is called epilepsy. Sources: https://dogtime.com/dog-breeds/pomeranian#/slide/1 https://www.dogzhealth.com/pomeranian-health-problems/ http://cdn.akc.org/Marketplace/Health-Statement/Pomeranian.pdf Photo credit: https://pixabay.com/photos/dog-pomeranian-cute-animal-canine-1113398/
Feline Immunodeficiency Virus (FIV): Virology, Diagnosis & Management
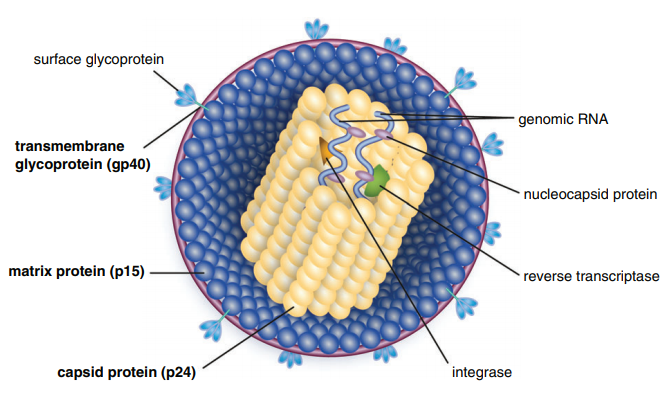
Table of Contents Maigan Espinili Maruquin 1. Introduction: The Scientific and Clinical Significance of FIV 1.1 Historical Discovery of FIV (1986) and Its Relevance as an HIV Model Feline Immunodeficiency Virus (FIV) was first recognized in 1986, when domestic cats in California presented with severe immunodeficiency syndromes remarkably similar to human AIDS. In 1987, Pedersen, Ho, and colleagues successfully isolated the virus, identifying it as a novel feline lentivirus and establishing the foundation for a new model of lentiviral immunopathogenesis (Pedersen, Ho et al. 1987). Initial observations emphasized that affected cats showed clinical signs of profound immune dysfunction—fever, lymphadenopathy, recurrent infections—but were negative for Feline Leukemia Virus (FeLV), differentiating this syndrome from previously known retroviral diseases in cats (Harbour, D.A. et al. 2004). Because FIV belongs to the Lentivirus genus within Retroviridae, similar to Human Immunodeficiency Virus (HIV), it was subsequently named in parallel with HIV. The biological, genomic, and pathological similarities between FIV and HIV—including their shared tropism for CD4⁺ T lymphocytes, slow progression, and lifelong persistence—positioned FIV early on as an important natural animal model for studying human AIDS (Elder & Phillips 1995; Miller, Cairns et al. 2000; Troyer, Pecon-Slattery et al. 2005; Hosie, Techakriengkrai et al. 2017). To this day, FIV remains a critical comparative model for HIV vaccine development, antiviral therapy research, and studies of lentiviral immune dysfunction. 1.2 Classification as a Lentivirus and Early Pathogenesis Studies FIV is a member of the Lentivirus genus, characterized by chronic, progressive infections and slow viral replication cycles. Like HIV, FIV integrates its genome into the host’s DNA to form a provirus, resulting in lifelong infection (Bendinelli, Pistello et al. 1995; Westman, Malik et al. 2019). Three Major Clinical Stages of Infection FIV infection progresses through a series of well-defined clinical stages that mirror HIV pathogenesis: Acute (Primary) Infection Occurs 1–6 weeks post-exposure and may last 1–4 weeks. Common findings include:• transient fever, lethargy, dullness• generalized lymphadenopathy• transient neutropenia• anorexia, diarrhea• mild upper respiratory signs(Ishida & Tomoda 1990; Bendinelli, Pistello et al. 1995) During this phase, the thymus and lymphoid tissues are important early replication sites where lesions may appear within 4 weeks (Harbour, D.A. et al. 2004). Asymptomatic (Latent) Phase Often lasts 1.5–2 years or longer.• Cats appear healthy but remain persistently infected.• Viral replication continues at low levels.• Subclinical chronic inflammatory lesions may occur (Harbour, D.A. et al. 2004). Terminal Immunodeficiency Phase (Feline AIDS; FAIDS) Characterized by:• severe immune collapse• high viral replication• chronic secondary infections• neoplasia (especially lymphomas)• neurological abnormalities(Pedersen 1993; Bendinelli, Pistello et al. 1995; Westman, Malik et al. 2019) These stages reflect lentiviral dynamics nearly identical to HIV progression in humans. 1.3 Distinction Between FIV and FeLV in Naturally Infected Cats Although both FIV and FeLV are important feline retroviruses, they differ significantly in classification, transmission, and pathogenesis: Classification and Molecular Differences FIV: Lentivirus; elongated morphology; Mg²⁺-dependent reverse transcriptase (Harbour, D.A. et al. 2004). FeLV: Gammaretrovirus; Mn²⁺-dependent reverse transcriptase. Transmission FIV: Primarily transmitted via bite wounds during fighting or mating aggression (Perharic, Bidin et al. 2016; Miller, Boegler et al. 2017). FeLV: Transmitted via casual contact: grooming, shared bowls, and vertical passage. Clinical Outcome FIV:• Lifelong persistent infection• Slow immunodeficiency progression• Many cats live normal or near-normal lifespans with appropriate care FeLV:• More acutely pathogenic• Causes immunosuppression, anemia, lymphoma• Can manifest as abortive, regressive, or progressive infection These differences require distinct diagnostic and management approaches. 1.4 Why FIV Remains a Critical Feline Pathogen in Modern Veterinary Medicine Global Prevalence and Risk Factors FIV prevalence varies with geography, lifestyle, and demographics:• 2.5–5 percent among healthy cats in North America• Up to ≥15 percent in high-risk populations (Bendinelli, Pistello et al. 1995)• Higher in older, free-roaming, intact male cats due to aggressive behaviors Pathogenesis: Progressive Immune Dysfunction FIV induces a chronic decline in immune function via:• depletion of CD4⁺ T cells• inversion of the CD4:CD8 ratio• chronic immune activation• susceptibility to secondary infections These immune abnormalities closely mirror HIV-induce immunopathology. Neoplastic and Neurological Sequelae FIV markedly increases the risk of neoplasia, especially B-cell lymphosarcoma.Neurological manifestations include:• seizures• altered behavior• dementia• ataxia(Ishida & Tomoda 1990; Bendinelli, Pistello et al. 1995) Transmission Challenges Although FIV is shed in saliva, deep bite wounds remain the dominant transmission route. Viral RNA, DNA, and antibodies have been detected in saliva and oral tissues (Yamamoto, Sparger et al. 1988; Pedersen, Yamamoto et al. 1989; Miller, Boegler et al. 2017). Ethical and Clinical Implications Because many FIV-positive cats remain healthy for years:• A positive test result must never be the sole basis for euthanasia (Harbour, D.A. et al. 2004).• Appropriate counseling is required to prevent mismanagement.• Early diagnosis enables interventions that improve longevity and quality of life. Importance in HIV Research The strong biological parallels between FIV and HIV continue to position FIV as a unique model for:• immune dysfunction• neurological disease• vaccine development• antiretroviral therapy testing(Hosie, Techakriengkrai et al. 2017) 2. Virology and Molecular Biology 2.1 Viral Structure Feline Immunodeficiency Virus (FIV) is a member of the Lentivirus genus within the Retroviridae family. Mature virions measure approximately 100–110 nm in diameter and contain a characteristic cylindrical nucleocapsid core. The virion is enclosed by a host-derived lipid bilayer acquired during budding from the plasma membrane of infected cells. Embedded within this lipid envelope are short surface glycoprotein spikes that are fundamental to viral infectivity and immune recognition. The viral Env glycoprotein precursor undergoes proteolytic cleavage to produce two mature subunits: Surface unit (SU, gp95) Transmembrane unit (TM, gp40) These subunits are non-covalently associated in a trimeric structure within the viral membrane. Together, they mediate: Receptor recognition and binding Membrane fusion and viral entry Immune evasion and antigenicity, serving as major targets for neutralizing antibodies The gp40 transmembrane glycoprotein is a key antigen detected by many point-of-care diagnostic kits, underscoring its immunological relevance. Within the core, the virion contains two identical positive-sense RNA strands assembled with nucleocapsid proteins, essential viral enzymes, and structural components. This genome–nucleoprotein complex is enclosed by the capsid protein (CA, p24), itself surrounded by the matrix protein (MA, p14)
Canine babesiosis
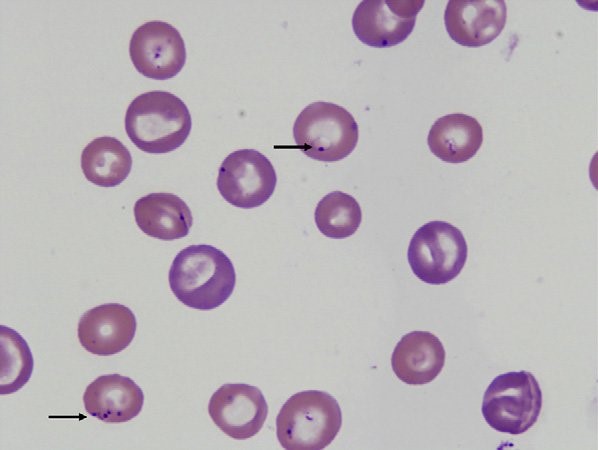
Canine babesiosis Robert Lo, Ph.D, D.V.M Canine babesiosis occurs worldwide and results from infections with a variety of Babesia spp., tick-borne hemoprotozoa. The disease was first described in cattle with hemolytic anemia in 1888 by a Rommanian bacteriologist, Victor Babes (Babes, 1888). Babesiosis is one of the most important tick-borne infectious diseases of domestic and wild mammals and still poses significant diagnostic and therapeutic challenges for veterinary practitioners around the world. More than 100 Babesia spp. were reported in vertebrate hosts (El-Bahnasawy et al., 2002). With the expansion of tick habitats, the spread of parasites into new geographical areas has been an increasing problem worldwide. The Babesia genus belongs to the order Piroplasmida in the phylum Apicomplexa and can be seen as non-pigment forming pear or signet-ring shaped organisms in mammalian erythrocytes. Asexual reproduction occurs in canine erythrocytes while the sexual conjugation and the sporogony stages of their life-cycles occurs in a variety of hard ticks, which can transmit the organism transovarially. Based on their morphology, Babesia are classified into the small Babesia group (trophozoites of 1.0-2.5 µm; including B. gibsoni, B. microti, and B. rodhaini), and the large Babesia group (2.5-5.0 µm; including B. bovis, B. caballi, and B. canis). Etiology and epidemiology Dogs are mainly infected by two species of Babesia: B. canis and B. gibsoni though they can also be infected by several other species of Babesia. Babesia canis has a piriform (teardrop) shape and frequently more than one merozoite is found in a single erythrocyte (Fig. 1). Babesia gibsoni is more pleomorphic (usually oval or signet-ring shapes) (Fig. 2). Fig. 1. Two pear-shaped Babesia canis organisms in an erythrocyte. (Duh et al., 2004) Fig. 2. Babesia gibsoni in erythrocytes in a blood smear stained with modified Wright technique. (Trotta et al., 2009) Babesia canis was further categorized into three subspecies (B. canis canis, B. canis rossi, B. canis vogeli) on the basis of cross-immunity, serological testing, vector specificity and molecular phylogeny (Uilenberg et al., 1989). These three subspecies of babesia are significantly different in their clinical presentation, geographical distribution and vector specificity. With the advent of molecular phylogenetic analysis, in particular that of the 18S rRNA gene, these subspecies are now considered to be separate species (Carret et al., 1999; Lack et al., 2012; Zahler et al., 1998). Recently an unnamed fourth “large” Babesia sp. (coco) has been found in dogs in North Carolina in the US (Birkenheuer et al., 2004) and has caused babesiosis in immunocompromised dogs (Sikorski et al., 2010). The small Babesia are more genetically closer to Theileria spp. than to Babesia spp. based on study of the 185 rRNA gene locus. Babesia canis, transmitted by Dermacentor reicultatus, is the most common pathogen of canine babesiosis in temperate regions of Europe and has been reported sporadically around the world (Solano-Gallego wt al., 2011). Most of clinical cases are reported in spring and autumn, which is associated with the seasonal activity of tick vector (Solano-Gallego et al., 2011; Matijatko et al., 2012). Babesia vogeli, transmitted by Rhipicephalus sanguincus, is a less pathogenic species and is found not only in tropical and subtropical regions but also in colder areas (Cassini et al., 2009). Babesia rossi, transmitted by Haemaphysalis elliptica (syn. Haemaphysalis leachi) (Penzhorn, 2011), is the most virulent species among large Barbesia species and is endemic in southern Africa but has been reported in other regions of eastern and southern Africa (Oyamada et al., 2005). Babesia gibsoni, a virulent parasite in dogs of all ages, is endemic in Asia and occurs sporadically in the rest of the world. Ticks of the complex R. sanguineus may serve as potential vectors for B. gibsoni, at least in Europe, while in Asia, its main distribution range is attributed to transmission by the tick Haemaphysalis longicornis (Hatta et al., 2013; Iwakami et al., 2014). In addition, B. gibsoni can be transmitted by blood exchange when dogs fight (Irwin, 2009). Transmission Babesia spp. are mainly transmitted through tick bites and can infect a wide variety of domestic and wild animals as well as humans (Schnittger et al., 2012). Hard ticks are the main vectors for Babesia spp.; within the tick, Babesia spp. undergo the sexual stage in the tick gut is followed by sporogony in its tissues. The parasite then reaches the tick salivary glands. A blood meal will ultimately transmit the sporozoites from the tick’s salivary gland to their new vertebrate host (Chauvin et al., 2009), where the protozoan undergoes asexual replication (merogony) within the red blood cells. Babesia spp. are transmitted both transstadially and transovarially (Chauvin et al., 2009). Pathogenesis and clinical signs After sporozoites enter the red blood cells, Babesia multiply via repeated binary fission, resulting in up to 16 merozoites. The parasites induce FLP (fibrinogen like proteases) that cause the red blood cells to become sticky, resulting in capillary sludging. Parasitized cells are sequestered in the spleen, and extravascular and intavascular hemolysis occurs. The incubation period following tick transmission is 10-21 days. The clinical picture is similar for all Babesia infections, whether they involve large or small Babesia. Pathogenicity is more severe in young dogs, immunosuppressed dogs, heavily parasitized dogs, and when there is exposure to a virulent strain or concurrent infection (Hunfeld et al., 2008; Matijatko et al., 2012; Schetters et al., 1997; Solano-Gallego L et al., 2008). Infected dogs may exhibit either peracute, acute, or subclinical signs of disease (Freeman et al., 1994; Jacobson, 2006; Wlosniewski et al., 1997). Peracute signs include acute onset of hypotensive shock, vasculitis, extensive tissue damage, hypoxia, and death. Signs of acute disease include fever, lethargy, hemolytic anemia, thrombocytopenia, splenomegaly, lymphadenopathy, icterus, and hemoglobinuria. Less common signs include ascites, peripheral edema, ulcerations, stomatitis, gastroenteritis, CNS signs, acute renal failure, and rhabdomyolysis. Acute infections of virulent strains of B. canis have been associated with induction of the systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) secondary to massive immune
Feline corona virus (FCoV) and Feline infectious peritonitis (FIP)
Feline corona virus (FCoV) and Feline infectious peritonitis (FIP) Andy Pachikerl, Ph.D Introduction: Feline coronavirus (FCoV) is a widely known positive-stranded RNA virus is infecting many cats worldwide (Satoshi, Takehisa and Motonobu 2012). This virus belongs to the species Alphacoronavirus 1 of the genus Alphacoronavirus from within the virus family Coronaviridae (Antoniw and Adams 2013). Alphacoronavirus 1 also includes the canine coronavirus (CCoV), which has been previously reported (link) and the porcine transmissible gastroenteritis coronavirus (TGEV) (Satoshi, Takehisa and Motonobu 2012). There are just two different forms of FCoV namely, the FECV (feline enteric coronavirus) that infects the intestines and FIPV (feline infectious peritonitis virus) that causes the disease feline infectious peritonitis (FIP). Feline coronavirus can typically be found in feces of infected cats and it can be transmitted to healthy cats via coming in contact by means of the fecal-oral route (Hartmann, Feline infectious peritonitis 2005). In environments with multiple cats, the transmission rate is higher as compared to a single-cat environment (Satoshi, Takehisa and Motonobu 2012). The virus is actually symptomless and insignificant until specific mutations occur that can cause complications such as FIP (Satoshi, Takehisa and Motonobu 2012). FIPV is the complication that can be the result of FCoV and causes FIP in cats, for which treatment is generally symptomatic and palliative only. The drug GS-441524 shows promise as an antiviral treatment for FIP, but now it’s still under further research development. Virology Feline enteric coronavirus (FECV) This is caused when the coronavirus becomes prominent in the mature gastrointestinal epithelial cells i.e. enterocytes, brush border, microvilli and villi of the cat (Rottier Peter, et al. 2005). This intestinal infection can show some outward symptoms and is usually chronic. The virus is excreted in the faeces of the symptomless carrier and can solely be detected by polymerase chain reaction (PCR) of faeces or by PCR testing of rectal samples. Cats that are raised in group can infect one another or each other with various strains of the virus. Some cats do show resistance to the virus and are not infected or even become a carrier, while others may become a FECV carrier (Rottier Peter, et al. 2005). Carriers may heal spontaneously but acquired immunity may be short and they may go on to being reinfection. Usually within a few weeks, if they are living in a group with healthy but persistent, excretory carriers; some cats will never heal, and the excretory phase remain permanent. Feline infectious peritonitis (FIPV) and Feline infectious peritonitis (FIP) The virus can further complicate into what is known as FIPV when random errors occur during when the virus infects an enterocyte, causing the virus to mutate from FECV to FIPV (Rottier Peter, et al. 2005). FIPV causes lethal, incurable disease such as: feline infectious peritonitis (FIP). Prior to being domesticated, cats are solitary animals and do not like to share space (i.e. hunting areas, rest areas and defecation sites). Domestic cats living in group therefore have a much higher epidemiological risk of mutation. After this mutation, the FCoV acquires a tropism for macrophages while losing intestinal tropism (Rottier Peter, et al. 2005). Regardless of the source of FIPV and uncertainty about the significance of genetic differences, the relationship between virulence and macrophage/monocyte tropism has been firmly established (Pedersen 2009). While both FIPV and FECV may cause viremia (Gunn-Moore, Bruffydd-Jones and Harbour 1998, Febr, et al. 1996), only FIPV replicates in macrophages and causes the disease (Vennema, et al. 1998, Stoddart and Scott 1989). Complex immune reactions between the virus, antiviral antibodies, and complement cause disseminated vasculitis, which is the hallmark of FIP (Hartmann 2005, Pedersen 2009). In a large group of cats, n, the epidemiological risk of mutation is higher and expressed theoretically as: E = n2 – n. A house hosting 2 cats therefore has a risk of E = 2. When 4 kittens (6 in total) are born into this group, the risk increases from 2 to 30. Overcrowding increases the risk of mutation and conversion from FECV to FIPV, which constitutes a very high-risk factor for the development of FIP cases. FIP has been shown to develop in cats whose immunity is low, such as younger kittens, old cats, immunosuppressed due to viral-FIV (feline immunodeficiency virus) and/or FeLV (Feline leukaemia virus) and stress such as: separation and adoption (Rottier Peter, et al. 2005). The incidence of disease is bimodal, occurring most commonly in cats younger than 18 months and older than 12 years of age. There is a genetic component that contributes to the risk of developing FIP, thus littermates of kittens that have developed FIP are at increased risk. Unfortunately, there is no way to predict, out of a group of FCoV seropositive cats at risk for FIP, which ones are most likely to develop the disease. Infection of macrophages by FIPV is responsible for development of a fatal granulomatous vasculitis, or FIP (see granuloma). Development of FIP depends on two factors: virus mutation and low immunity where virus mutation depends on the rate of mutation of FECV to FIPV and the immune status depends on the age, gene pool and the stress level of the cat. High immune status will be more effective at slowing down the virus (Rottier Peter, et al. 2005). How does FCoV cause FIP? Infections with FCoV are usually limited to the intestinal tract, with very limited viral replication elsewhere. Strains of FCoV causing these infections are referred to as feline enteric coronavirus (or FECV). During infection, and while the virus replicates in the intestine, it undergoes spontaneous mutations. This leads to the development of different strains of the virus, and occasionally a strain may develop that has dramatically altered disease-causing potential – this viral strain is referred to as feline infectious peritonitis virus (FIPV). FIPV strains of FCoV differ from FECV in that they no longer replicate well in the intestine, but rather preferentially infect macrophages – one of the important cells of
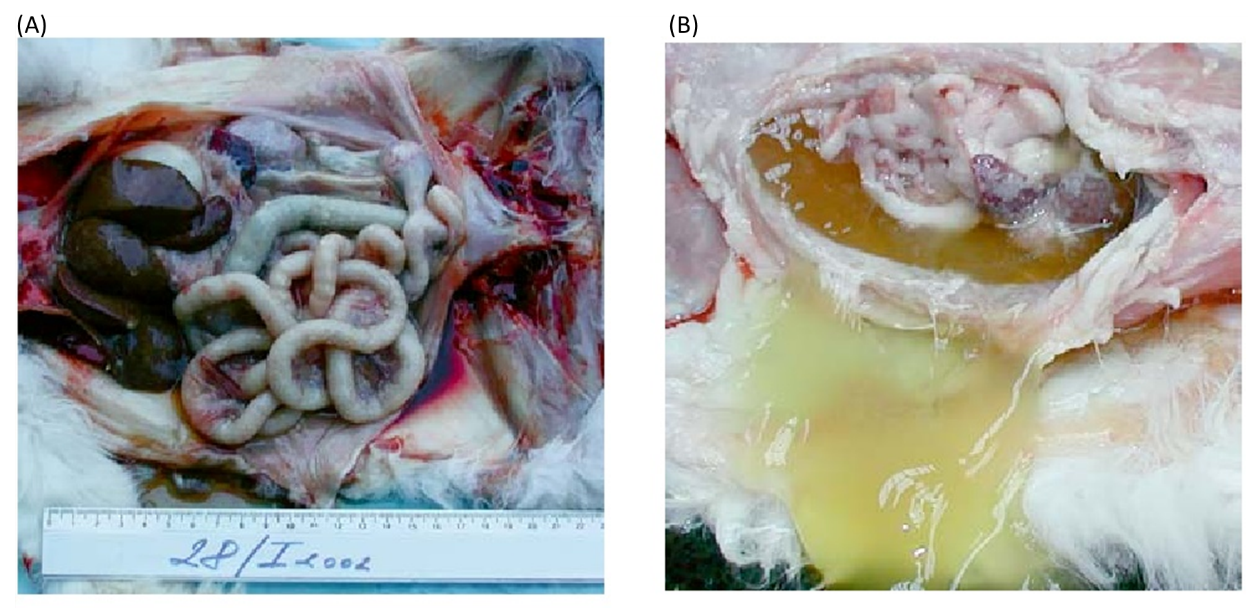
Giardia lamblia
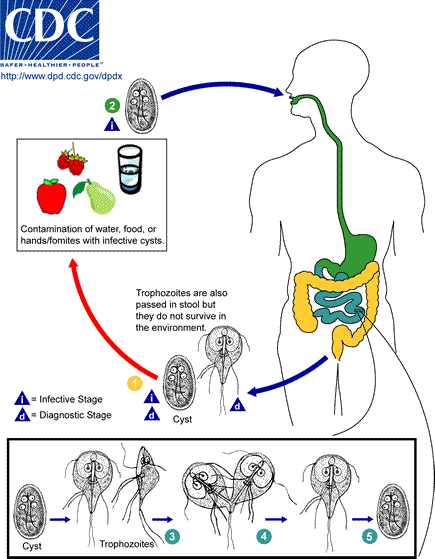
Giardia lamblia LIN, WEN-YANG (WESLEY), Ph.D Giardia lamblia (=G. intestinalis, =G. duodenalis) also called Giardia duodenalis, Giardia intestinalis and pear-shaped flagellate is a common and well-known anaerobic flagellated protozoan parasites colonize in human (or in canine) small intestines and cause diarrhea, stomach pain etc. Its character of parasitic zoonoses make them also infecting other mammalian such as mice, rabbits, birds, reptiles and amphibians. Giardia lamblia prefer to live in cold water like rivers in mountains, cold springs and contaminated pools, which became the major cause of diarrhea in the US. Traveling in the developing world, dinning food without proper cooking, changing diapers etc. would be major risk factors of Giardiasis. The stool tests is the major diagnosis tool. Discovery In 1681, the Dutch scientist Antonie van Leeuwenhoek first found giardia through microscope. In 1915, Giardia lamblia was officially named after scientist Alfred Mathieu Giard who further studied it. In 2010, Andersson et al. has sequenced Giardia’s genome and discovered its ~5000 genes with 11.7 million base pair building blocks. Cellular and physiological properties Giardia is a diplomonad with two nuclei and duplicate organelles follow by four associated flagella and without cytostomes, Golgi apparatus and mitochondria. However, they have a mitochondrial remnant, mitosomes, which take part in the maturation of iron-sulfur proteins rather than ATP synthesis. The life cycle of Giardia lamblia consist of reproductive phase and resting phase that present two different forms: a swimming trophozoite and an infective cyst (Figure 1). Genotyping of Giardia lamblia sub-classified eight genetic assemblages (from A to H). Among eight assemblages, A and B infect are the most dominate assemblages that infect wide range of species including human being. Various species of Giardia exist in different kind of hosts were identified by PCR or genetic tools include G. ardeae and G. psittaci from birds, G. agilis from amphibians, G. microti from voles and G. muris from other vertebrates. Epidemiology In 2013, WHO estimated that there were about 280 million people diagnosed with the Giardia infection around the world. The prevalence rate of Giardia in developed countries was 5% and over 20% among developing countries. In 2018, it present that 15,584 reported cases in 3–7% prevalence of the population in the US, especially high incidence in California, New York, Florida, and Wisconsin. Besides, the highest incidence months of giardiasis would be July, August, and September in the America. Furthermore, 23 of the 31 countries in the Europe had reported total 17,278 confirmed giardiasis cases in 2014. Thus, Giardiasis become one of the top 10 parasite diseases in human beings. Risk factors Travelers to countries where giardiasis is common. Contacting contaminated drinking water, lakes or rivers, animals who have the disease. Giardiasis often happened in the summer because of the higher activities rate in the wilderness. Pathophysiology Giardia exist in rivers, streams, wells and pools. It infect humans or animals by their contacting of contaminated foods, water, contaminated feces and sniffing unclean ground. It is reported that people (or pets) infected with Giardia may have no symptoms, but still spread the disease. Animals such as canines, cows, rodents, beavers, and sheep are also infecting targets of Giardia lamblia. Figure1. The life cycle of Giardia lamblia ( http://www.dpd.cdc.gov/dpdx/images/ParasiteImages/G-L/Giardiasis/Giardia_LifeCycle.gif ) Giardia could transform between cyst form and trophozoite form. It spread and infect host by the cyst form that can remain contagious in cold water up to 3 months. After arriving intestines, Giardia stick to intestinal wall and colonize in the gut by altering its appearance into trophoziote form. Furthermore, they would recover back to cyst form when excreting out with stools. Giardia is spreading through fecal-oral route by animals’ contacting of contaminated water and food. It is one of the most common waterborne outbreaks of diarrhea in the America. Giardia spread between people and animals (Figure 1). When sojourning in the gastrointestinal tract with the trophoziote form, Giardia would engage in inhibiting of brush border enzymes that involved assimilation of disaccharide sugars, altering microvillus’ morphology that cause poor absorption of nutrients and water, triggering apoptosis of intestinal epithelial cells and enhancing intestinal permeability. Eventually, they would cause several clinical symptoms include diarrhea and intestinal malabsorption with or without histological changes. For increasing intestinal permeability, Giardia lamblia would take several dedicated process such as assisting proliferation of crypt cells and using enzymes to degrade proteins on the villi of the brush border. The degraded proteins would likely lead to immunological recruitment and activation of host T lymphocytes on endothelial cells for removing injured cells. In addition, Giardia triggered cell apoptosis by downregulating of the anti-apoptotic Bcl-2 and upregulating of the proapoptotic Bax would facilitate breaking down intestinal barrier and enhancing permeability. They could also execute tactics of consuming all local arginine for decreasing the formation of the gas nitric oxide and protecting its own development. Signs and symptoms The parasitic disease caused from the infection of Giardia, called Giardiasis, also known as beaver fever. A 10% of Giardiasis can be temperate and showed no sign, which recovery by host’s immune function without extra treatments. Nevertheless, 90% infected hosts show apparent symptoms 2 days after infection and last 1 to 6 weeks. Giardia would make atrophy in the small intestinal villi that would lead to foul- smelling diarrhea and cause dehydration accompanied with malnutrition. Diarrhea is the most common and dominant sign (in both humans and animals) accompanied by other symptoms include excess gas, abdominal cramps, abdominal pain, weight loss, nausea, vomiting, bloody feces and fever. However, only about 15% of infected hosts would occur fever. Most canines would be less active instead of exhibiting fever. Lesser common cases shows signs beyond intestinal system as itchy skin, hives and swelling of the eyes and joints. Medication should be administrated as soon as possible. Chronic diarrhea could last for weeks or months if untreated. Symptoms such as post-infectious irritable bowel syndrome, lactose intolerance and food allergies may occur and remain even if Giardia were dealt with medication. Diagnosis Detecting antigens in stool specimens, which
Breed-related disease: Himalayan

John K. Rosembert The Himalayan cat is a medium-sized cat very similar in appearance to the Persian cat but distinguished by the points on the cats’ extremities (the facial mask, feet, ears, and tail) which results in a Persian-type cat with the coloring and deep blue eyes of the Siamese-patterned cat. The ideal Himalayan is a strong cat with excellent boning and musculature, a well-balanced cat, giving the impression of robust power. As with their Persian cousins, the Himalayan cat has two facial variations : traditional / doll-faced, or peke-faced (named after the Pekingese dog with squashed-looking features). The Himalayans make great indoor pets. They possess the best characteristics from the Siamese and the Persian. Their activity levels lay between that of the Siamese and Persian, so they’re equally happy to play as they are to relax, making them great family pets They are loyal and affectionate cats who require lots of attention and love but tend to play favorites among their owners. They are very social, sweet and intelligent, and have been known to be quite talkative. Here we bring you the most common Himalayan Cat Diseases & Conditions. Polycystic kidney disease. PKD is a condition that is inherited and symptoms can start to show at a young age. Polycystic Kidney Disease causes cysts of fluid to form in the kidneys, obstructing them from functioning properly. It can cause chronic renal failure if not detected . Look for symptoms like poor appetite, vomiting, drinking excessively, frequent urination, lethargy and depression. Breathing issues. Peke-faced cats have a compacted snout and airway and as a result, it may suffer shortness of breath or noisy breathing. Cherry eye . is a condition affecting the eye, causing the third eyelid to well and cause irritation It generally appears as a red mass (hence the name cherry) on the corner of the cat’s eye. It is treated with surgery. Progressive retinal atrophy. Refers to a family of eye conditions which cause the retina’s gradual deterioration. Night vision is lost in the early stages of the disease, and day vision is lost as the disease progresses. Many cats adapt to the loss of vision well, as long as their environment stays the same. Entropion. It is a condition that can occur in Persians and causes the eyelid to roll inwards, which can lead to irritation or injury of the eyeball. Signs include rubbing or scratching around the eye area. It can be treated surgically if necessary. Primary seborrhea. is a skin condition in which the skin becomes greasy, scaly and smelly due to the overproduction of skin cells. Sources: http://www.vetstreet.com/cats/himalayan#1_ugw20zmq https://bowwowinsurance.com.au/cats/cat-breeds/himalayan-cat/ Photo credit: https://www.shutterstock.com/ search / himalayan + cat https://www.wwwallaboutcats.com/best-food-for-himalayan-cats
