Vector-borne disease: Ehrlichia spp. infection
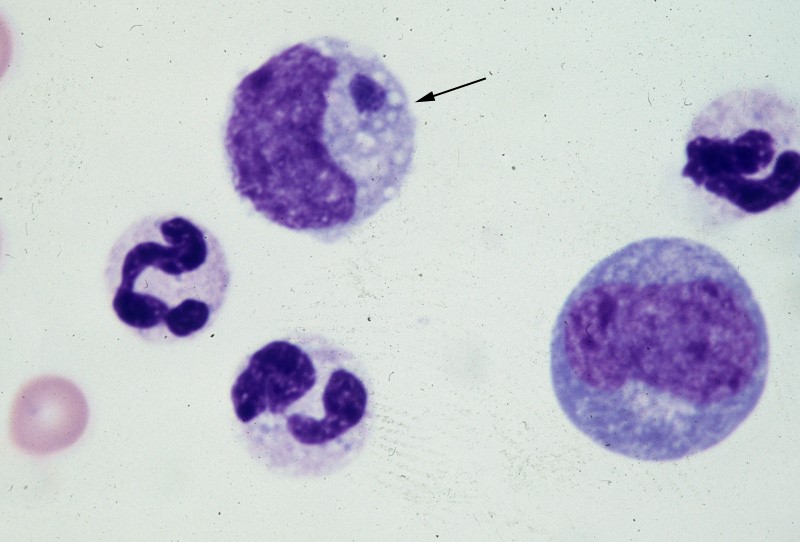
Vector-borne disease: Ehrlichia spp. infection Canine Ehrlichiosis Andy Pachikerl, Ph.D Introduction Ehrlichiosis is a disease of dogs, humans, livestock, and wildlife that is widely distributed around the world and is transmitted by tick vectors. The pathogen of the disease, Ehrlichia, was renamed and classified in 2001 according to the bacterial 16S RNA and groESL gene nucleic acid sequences, and is classified as Rickettsiales, Anaplasmataceae, Ehrlichia genus of bacteria (Allison and Little, 2013).) With global warming, the expansion of tick habitats and the prevalence of cross-border tourism, the chances of the disease spreading to non-endemic areas have increased. How is a dog infected with Ehrlichia? Ehrlichiosis is a disease that develops in dogs after being bitten by an infected tick. In the United States, E. canis is considered endemic in the southeastern and southwestern states, though the brown dog tick is found throughout the United States and Canada. (Photo credit: https://vcahospitals.com/know-your-pet/ehrlichiosis-in-dogs) Pathogens and transmission. Ehrlichia spp. are gram-negative, small, obligatory intracellular bacteria. There are currently three types of Ehrlichia spp.: not limited to dogs: Elyse infection, dogs as hosts: E. canis, E. chaffeensis, and E. ewingii. E. canis can infect dogs causing monocytic ehrlichsis (canine monocytic ehrlichsis, CME). Cells most commonly infected by E. canis are monocytes and lymphocytes (Figure 1). CME occurs mainly in tropical and subtropical regions, but there are also cases of infection in other regions. E. chaffeensis infects single-core balls of dogs and humans, mainly in North America, South America, Asia and Africa. E. ewingii is a zoonotic infectious disease that infects particulate white blood cells and is found mainly in North America, South America and Cameroon, Africa. E. ewingii causes human granulocytic ehrlichiosis (Bulleretal., 1999). E. chaffeensis infects humans known as human monocytic ehrlichiosis. E. canis also causes human infections (Maeda et al., 1987). Ehrlichia spp. life history is that of vector ticks and mammalian hosts. After sucking the blood of infected animals, the larvae transmit the disease to the new host via saliva when they bite and suck the blood of other animals. E. canis, E. chaffeensis and E. ewingii have been shown to mediate life cycle transmission in an intermediary hosts that are usually arthropods. This have shown to stabilize its life cycle transmission, which is also known as Transstadial transmission in arthropods, and blood transfusions or bone marrow may also cause the spread of the disease. The main vector arthropods in E. canis are Rhipicephalus sanguineus and Dermacenter variabilis even though the main host are canines including domestic dogs. Other canine family it can infect are wolves, coyotes, and foxes. The other species, E. chaffeensis and E. ewingii consist of hosts from the arthropod family such as Amblyomma americanum, a main vector and other arthropods such as Haemaphysalis, Dermacentor, and Ixodes. E. chaffeensis usually infects white-tailed deer, and E. ewingii to hosts that are likely deer and dogs. Figure 1. Ehrlichia canis in the monocyte (arrow) (Wright’s stain, 1000x) (Source: http://www.eclinpath.com/ngg_tag/infectious-agent/nggallery/page/9) Clinical symptoms. The clinical symptoms and severity of Ehrlichiosis depend on the type of Elysian infection and the host’s immune response. The course of E. canis infection in dogs can be divided into acute, subclinical and chronic, but in naturally infected dogs it is not easy to distinguish between these three stages. If there is a co-infection with other pathogens, it will also aggravate the severity of the disease. The acute period lasts about three to five weeks and can cause fever, poor spirits, loss of appetite, swollen lymph nodes and swollen spleen. Increased eye secretions, pale mucosa, bleeding disorders (bleeding spots, or runny nosebleeds), or neurological symptoms caused by meningitis. Vomiting, diarrhea, lameness, reluctance to walk, stiff pace, and leg or scrotum edema. The most easily observed hematological abnormalities are white blood cell reduction, platelet reduction, and anemia. When clinical symptoms disappear, they are often accompanied by subclinical periods that last for several years (Waner et al., 1997). Although some cases can be fatal, others heal on their own. When a dog is unable to clear the pathogen of infection, the course of the disease develops into a subclinical persistent infection to become a primary dog. Some infected dogs enter a chronic period. During the chronic period, symptoms and hematological abnormalities, including platelet reduction, anemia and total blood cell reduction, all relapse and become more severe than it was during acute periods. When the severity maximizes in a few cases, the dogs will not respond to antibiotic treatment and ceased to heal, and eventually they die from heavy bleeding, severe weakness, or secondary pathogenic infections. Many times, when an infected dog that has contracted E. chaffeensis, the diagnoses points out to other pathogens since there are little information as to E.chaffeensis and dog infections also, the clinical symptoms are similar to that of infection with E. canis. Symptoms include easy bleeding (bleeding spots, blood urine or nosebleeds), vomiting, and swollen lymph nodes. The most common symptom of infection with E. ewingii is fever. Other symptoms include lameness, multiple arthritis, terminal edema, swollen lymph nodes, reduced platelets and anemia, some of which can cause neurological symptoms in dogs. Diagnosis. Ehrlichiosis can be diagnosed by microscopy, serology, or PCR. Diagnosis is complicated when other arthropod-mediated pathogens are used. Blood smears have been observed to infect blood cells to help diagnose the disease. However, this method is time-consuming and can only be observed in a small number of cases during acute infection, so it is not a reliable diagnostic method. Serological examinations are often used to assess Ehrlichiosis. Detection of IgG antibody deposits indicated that dogs had been exposed to pathogens, and two serological examinations two weeks apart during the acute period showed an increase in antibody force prices. Caution should be exercised when a single test result is judged, as healthy dogs may also be antibody positive. Antibodies cannot be detected in the early stages of infection. Additionally, antibodies produced during and after an infection with E.canis, E. chaffeensis, or
Case study: Canine non‐epitheliotropic CD4‐positive cutaneous T‐cell lymphoma: a case report
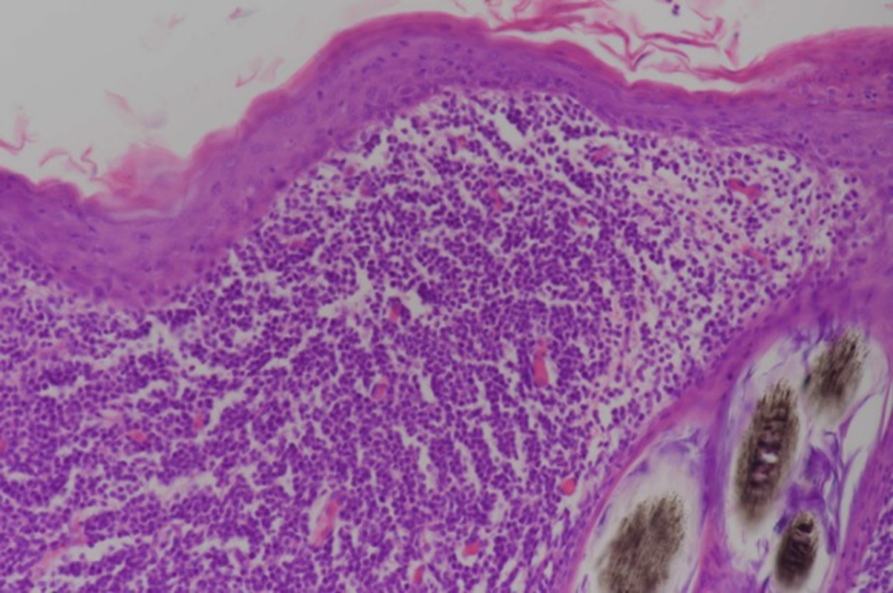
Case study: Canine non‐epitheliotropic CD4‐positive cutaneous T‐cell lymphoma: a case report Robert Lo, Ph.D, D.V.M A 5‐year‐old, spayed female French Bulldog presented with multiple papules on the skin of the scapular area. Histopathological examination of skin biopsy specimens showed proliferated small lymphoid cells in the superficial dermis and in the area around the hair follicle. Immunohistochemical examination revealed that these cells were positive for CD3, CD4 and TCRαβ antibodies, but negative for CD1c, CD8α, CD8β, CD11c, CD20, CD45RA, CD90, MHC-II and TCRγδ antibodies. In addition, CD45 is highly expressed, and proliferation is very low. The genetic recombination test of the T cell receptor G chain detects the proliferation of recombinant clones. Skin lesions were removed by surgery because of progressing to the outside of the forelegs. The postoperative clinical course was good, and no recurrence was observed until the dog died in a traffic accident about a year later. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6498901/ Figure 1 Clinical features of the dog. Multiple papules are present on the right scapular area. Figure 2 Histopathological features of the lesion. Small lymphoid cells are proliferative at the superficial dermis and the perifollicular areas. Figure 3 Immunohistochemical analysis via the avidin‐biotin‐peroxidase complex method. Dense infiltration of CD4‐positive small lymphoid cells is evident at the superficial dermis. Bar = 200 μm.
Case study: Coinfection with Tritrichomonas foetus and Giardia duodenalis in Two Cats with Chronic Diarrhea
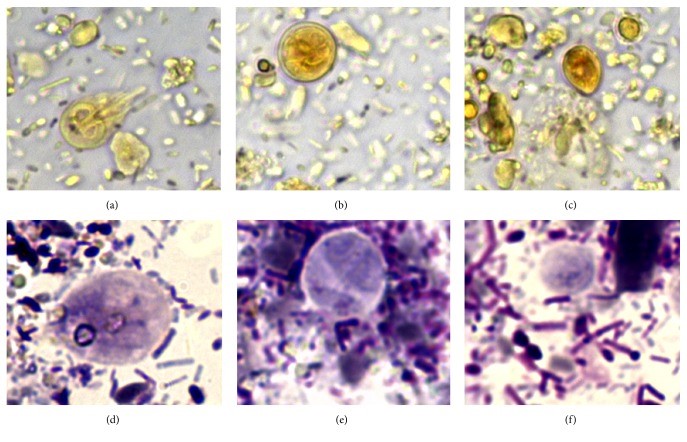
Case study: Coinfection with Tritrichomonas foetus and Giardia duodenalis in Two Cats with Chronic Diarrhea Robert Lo, Ph.D, D.V.M A mixed infection of Tritrichomonas foetus and Giardia duodenalis was confirmed in two 6-year-old Maine Coon cats. One of the cats had a history of chronic liquid diarrhea and several treatment failures. Both cats observed G. duodenalis and trichomonas from the fecal smears, and the infection of T. foetus was also confirmed by RT-PCR. The cat recovered completely after taking ronidazole treatment. In refrigerated stool specimens collected from cats with chronic diarrhea, drop-shaped trichomonad pseudocysts, which are smaller than the cysts of G. duodenalis, were detected. When the pseudocysts are stained with Lugol’s solution or Giemsa, they appear brown or light blue, respectively, and their morphological characteristics are similar to those of bovine T. foetus in vitro. It is worth noting that the pseudocysts in feline trichomonads may be a way for the protozoa to fight against unfavorable environments. Clinicians detected pseudocysts in refrigerated stool, which may be a useful clues to the diagnosis of this disease. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6005279/ Figure 1 Fecal smears of a 6-month-old female Maine Coon cat with chronic liquid diarrhea, stained with Lugol’s solution (a–c) and Giemsa stain (d–f); showed (a) and (d) Giardia duodenalis trophozoites ; (B) and (e) a Giardia duodenalis cyst; (c) and (f) drop-shaped trichomonads (630x). Fecal smears from a 6-month-old female Maine Coon cat with chronic liquid diarrhea stained with Lugol’s solution (a–c) and Giemsa stain (d–f); (a) and (d) showed Giardia duodenalis trophozoite; (b) and (e) showed a Giardia duodenalis cyst; (c) and (f) showed drop-shaped trichomonads (630x). Figure 2 Trichomonads in fecal smear from the cat with diarrhea. Arrow heads in (a) indicate anterior flagella emerging from the trophozoite, while arrow heads in (b) indicate undulating membrane (1000x). Figure 3 Typical morphology of trichomonads observed in saline solution-diluted fresh fecal smear from the cat with diarrhea. Arrow heads in (a) and in (b) indicate anterior flagella and undulating membrane, respectively (630x). Figure 4 Drop-shaped unidentified elements in fecal smears stained with Lugol’s solution (a) and Giemsa stain (b-c). Arrow heads in (a) indicate an internal oval structure (400x). Arrow heads in (b) indicate a curved linear structure (1000x). Arrow heads in (c) indicate an undulated portion of the surface (1000x).
Toxoplasmosis In Cats: A Review
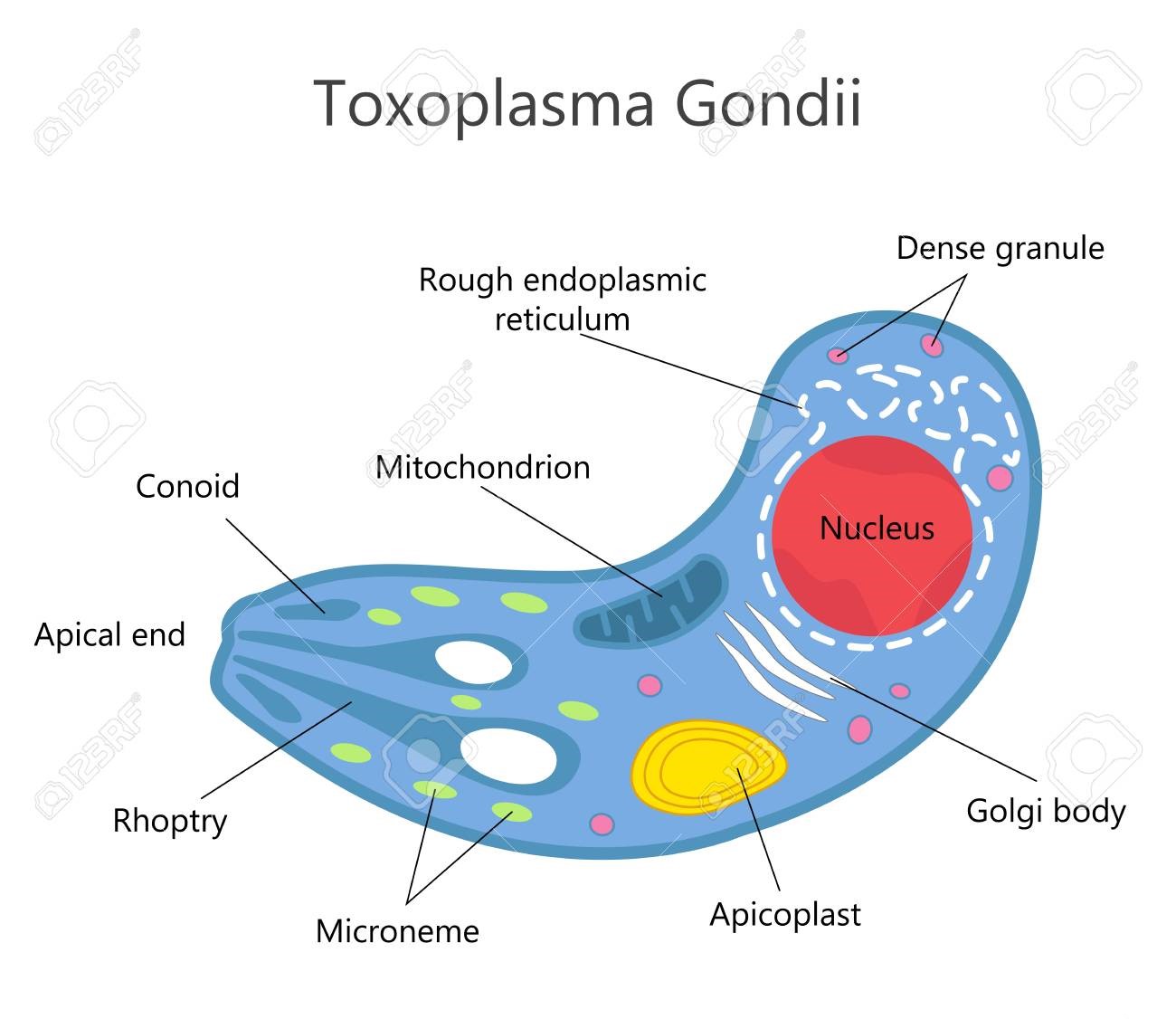
Toxoplasmosis In Cats: A Review. Maigan Espinili Maruquin Structure and Replication Fig. 01 Structure of Toxoplasma gondii (https://www.123rf.com/photo_81668845_stock-vector-toxoplasma-gondii-structure-.html) The family Felidae is the only animal species that hosts infective oocysts of Toxoplasma gondii and passes through their feces, however, this parasite infects most species of birds and mammals (Elmore, Jones et al. 2010). This pathogen is an obligate intracellular coccidian parasite an can infect warm-blooded animals, including people (Hartmann, Addie et al. 2013) (Dubey JP, 2005; Dubey JP and Lappin MR, 2006). The complex life cycle of T. gondii undergoes three distinguished stages. The tachyzoites, formerly called the trophozoite or endozoite, is the active multiplying stage and most likely to cause clinical disease and spread to almost all organs. The next stage is the bradyzoite stage where multiplication is slow and usually within a tissue cyst, leading to a life-long chronic infection. This stage penetrates the small intestine epithelial cells. Finally, the oocysts, which are excreted and shed in feces of infected felid, are the result of sexual reproduction within the intestine and constitute the environmentally resistant stage. (Dubey JP, 2005; Dubey JP and Lappin MR, 2006) (Dabritz, Gardner et al. 2007, Dabritz, Miller et al. 2007, Hartmann, Addie et al. 2013, Wyrosdick and Schaefer 2015, Calero-Bernal and Gennari 2019). Oocysts are non- infectious when excreted in feces but begin to become sporulate after 1-5 days of exposure to air and moisture. These are round to oval in shape and around 10 x 12 μm in size. Most naïve cats who get infected take 3–10 days of ingestion of tissue cysts to complete the cycle (Hartmann, Addie et al. 2013) (Dubey JP, 2005; Dubey JP and Lappin MR, 2006). Infection/ Pathogenesis The very first case of toxoplasmosis in cats was diagnosed from a domestic cat in Middletown, NY, in 1942 (Olafson and Monlux, 1942; Dubey, 2008)(Wyrosdick and Schaefer 2015). Generally, most cats at 6 to 10 weeks were detected to have antibodies to T. gondii while maternally transferred antibodies (MTAs) disappear by 12 weeks of age. Seropositivity increases with the age and varies according to the cat’s lifestyle (like hunting food) (Dubey, J.P., 2010)(Dubey, Cerqueira-Cézar et al. 2020). The oocysts were noted to remain infectious in the environment for at least 12 months (Hutchison 1965, Wyrosdick and Schaefer 2015). Generally, transmissions of the parasite are congenital infection, ingestion of the infected tissue, and ingestion of oocyst-contaminated food or water (Dubey JP and Lappin MR., 2006) (Hartmann, Addie et al. 2013). Most likely, congenitally infected kittens show clinical signs while post-natal infections are usually through ingestion of infected tissue cysts and in some cases, oocysts (Dubey and Jones 2008, Elmore, Jones et al. 2010). Queens giving birthe to infected kittens during gestation can become infected transplacentally or via suckling (Dubey JP, et al., 1996) (Calero-Bernal and Gennari 2019) The most common method of feline infection due to predation of intermediate hosts is tissue cyst ingestion where shedding occurs in 3 to 5 days, while ingestion of tachyzoites takes 8- 10 days, and 21-24 days after ingestion of oocysts (Dubey, J.P., 2010) (Schares, Vrhovec et al. 2008, Wyrosdick and Schaefer 2015). Clinical Signs Feline toxoplasmosis develops clinical signs rarely but causes inflammation and tissue necrosis from intracellular growth of tachyzoites (Dubey JP and Lappin MR, 2006)(Hartmann, Addie et al. 2013). It frequently results to hepatitis, pneumonia, and encephalitis with signs of ascites, lethargy, and dyspnea while infected adults do not show specific clinical signs (Brennan A, et al, 2016) (Dubey and Carpenter 1993, Calero-Bernal and Gennari 2019). Moreover, observations also showed extra- intestinal enteritis (Cohen, Blois et al. 2016) and inflammatory intestinal disease (Peterson, Willard et al. 1991). Tissues that are most commonly affected are the central nervous system, the muscles, the lungs, and the eyes. Infected cats show neurological signs, muscle hyperesthesia, jaundice, diarrhea, fever, depression, anorexia, vomiting, paresis, dermatitis and weight loss (Dubey JP and Lappin MR, 2006) (Hartmann, Addie et al. 2013, Dubey, Cerqueira-Cézar et al. 2020). When severe respiratory and neurological signs were observed, it’s usually fatal (Dubey and Carpenter 1993). Diagnosis Diagnosis in cats for toxoplasmosis include ante-mortem fecal examination for oocysts and serologic testing (Johnson, Tinker et al. 2009, Elmore, Jones et al. 2010). However, geographical location may influence differential diagnosis (Calero-Bernal and Gennari 2019). The shedding of the oocysts of an infected cats may only be once in their lifetime (Elmore, Jones et al. 2010) which can be diagnosed in their fecal samples via microscopy (Hartmann, Addie et al. 2013). However, there is low probability of finding oocysts in the fecal samples of infected cats and there is a confusing morphological resemblance of the T. godii oocysts to other coccidian like Hammondia hammondi and Besnoitia spp (Elmore, Jones et al. 2010). Therefore, molecular and bioassay techniques can be used to distinguish them while only mouse bioassay is the definitive confirmation method (Dubey 2009, Elmore, Jones et al. 2010). In diagnosing T. gondii, confirmation is when the organism is found in body fluids or tissue (Hartmann, Addie et al. 2013). For tachyzoites detection, ante- mortem diagnosis in tissues and body fluids during acute illness may use cytology or polymerase chain reaction (PCR). A definitive diagnosis is when tachyzoites were detected rarely in blood but aqueous humour, lymph nodes, and transtracheal or bronchoalveolar lavage fluid can be used (Hartmann, Addie et al. 2013). On the other hand, antibodies of the IgM, IgG and IgA isotypes can be detected by immunofluorescence assay (IFA). For antibody- negative, cats are likely to shed oocysts while antibody- positive cats don’t shed oocysts wherein antibodies need 2–3 weeks to develop (Dubey JP, 2005)(Hartmann, Addie et al. 2013). Despite the development of immunofluorescence and ELISA tests, requirement for species-specific protein conjugate makes it limitedly used in veterinary diagnostics (Wyrosdick and Schaefer 2015). After comparing indirect hemagglutination test, latex agglutination test, Feldman dye test and modified agglutination tests, the aqueous humour resulted to be the most sensitive of
Heartworm in Cats
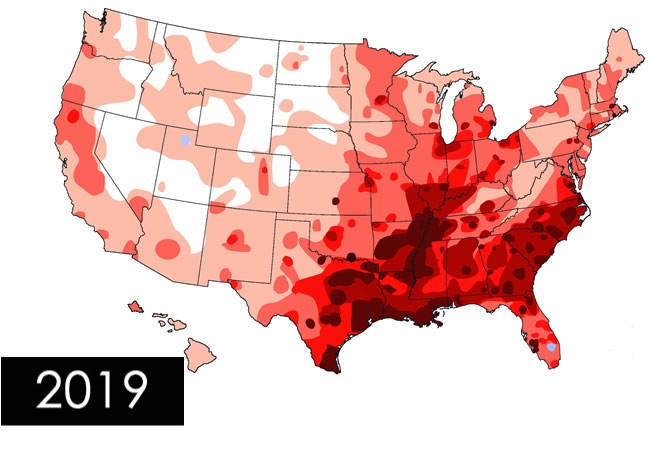
Heartworm in Cats Andy Pachikerl, Ph.D Introduction of Feline Heartworm Heartworm disease is a serious and potentially fatal disease in pets worldwide and it is caused by foot-long worms (heartworms) that dwells in the heart, lungs, and associated blood vessels of affected pets. This in turn cause severe lung disease, heart failure and damage to other organs in the body. Heartworm disease affects dogs, cats, and ferrets, but heartworms also live in other mammal species, including wolves, coyotes, foxes, sea lions and—in rare instances—humans. Because wild species such as foxes and coyotes live in proximity to many urban areas, they are considered important carriers of the disease (Ettinger, et al., 2010). Among mammalian definitive hosts, they are best adapted to domesticated and wild dogs. If untreated, their numbers can increase, and dogs have been known to harbour several hundred worms in their bodies. Heartworm disease causes lasting damage to the heart, lungs, and arteries, and can affect the dog’s health and quality of life long after the parasites are gone (2019). For this reason, prevention is by far the best option, and treatment—when needed—should be administered as early in the course of the disease as possible. Heartworm disease in cats is quite distinctive from heartworm disease in dogs. Since cat is an atypical host for heartworms, most worms in cats do not survive to the adult stage. Cats with adult heartworms usually have just one to three worms, and many cats affected by heartworms have no adult worms. Even if feline host of heartworm rarely occurs, cat heartworm disease often goes undiagnosed. Therefore, it is important to understand that even immature worms cause real damage in the form of a condition known as heartworm associated respiratory disease (HARD). Moreover, the medication used to treat heartworm infections in dogs cannot be used in cats, so prevention is the only means of protecting cats from the effects of heartworm disease (Society, 2007). Transmission Figure 1. Diagram from American Heartworm Society showing how the heartworm is spread and inter-transmission can occur via different host pets. The mosquito plays an essential role in the heartworm life cycle. Adult female heartworms living in an infected dog, fox, coyote, or wolf produce microscopic baby worms called microfilaria that circulate in the bloodstream. When a mosquito bites and takes a blood meal from an infected animal, it picks up these baby worms, which develop and mature into “infective stage” larvae over a period of 10 to 14 days (Johnstone, 1998). Then, when the infected mosquito bites another dog, cat, or susceptible wild animal, the infective larvae are deposited onto the surface of the animal’s skin and enter the new host through the mosquito’s bite wound. Once inside a new host, it takes approximately 6 months for the larvae to mature into adult heartworms. Once mature, heartworms can live for 5 to 7 years in dogs and up to 2 or 3 years in cats. Because of the longevity of these worms, each mosquito season can lead to an increasing number of worms in an infected pet (Knight, et al., 1998). Symptoms and signs of heartworm in cats Signs of heartworm disease in cats can be of two extremes: very subtle or plain out dramatic. Symptoms may include coughing, asthma-like attacks, periodic vomiting, lack of appetite, or weight loss. Occasionally an affected cat may have difficulty walking, experience fainting or seizures, or suffer from fluid accumulation in the abdomen. Unfortunately, the first sign in some cases is collapse of the cat, or sudden death (Society, 2007). How significant is my cat’s risk for heartworm infection? Figure 2. Diagram showing the severity of heartworms in the USA as shown by the American Heartworm Society Many factors must be considered, even if heartworms do not seem to be a problem in your local area. Your community may have a greater incidence of heartworm disease than you realize—or you may unknowingly travel with your pet to an area where heartworms are more common. Heartworm disease is also spreading to new regions of the country each year. Stray and neglected dogs and certain wildlife such as coyotes, wolves, and foxes can be carriers of heartworms. Mosquitoes blown great distances by the wind and the relocation of infected pets to previously uninfected areas also contribute to the spread of heartworm disease (this happened following Hurricane Katrina when 250,000 pets, many of them infected with heartworms, were “adopted” and shipped throughout the country). The fact is that heartworm disease has been diagnosed in all 50 states, and risk factors are impossible to predict. Multiple variables, from climate variations to the presence of wildlife carriers, cause rates of infections to vary dramatically from year to year—even within communities. And because infected mosquitoes can come inside, both outdoor and indoor pets are at risk. For that reason, the American Heartworm Society recommends that you “think 12:” (1) get your pet tested every 12 months for heartworm and (2) give your pet heartworm preventive 12 months a year. Diagnosis Heartworm disease is a serious, progressive disease. The earlier it is detected, the better the chances the pet will recover. There are few, if any, early signs of disease when a dog or cat is infected with heartworms, so detecting their presence with a heartworm test administered by a veterinarian is important. The test requires just a small blood sample from your pet, and it works by detecting the presence of heartworm proteins. Some veterinarians process heartworm tests right in their hospitals while others send the samples to a diagnostic laboratory. In either case, results are obtained quickly. If your pet tests positive, further tests may be ordered (Yin, 2007). Diagnosis period Heartworm infection in cats is harder to detect than in dogs, because cats are much less likely than dogs to have adult heartworms. The preferred method for screening cats includes the use of both an antigen and an antibody test (the “antibody”
Feline Calicivirus Review: Biology, Risks, and Care

Table of Contents 1. Introduction: Feline Calicivirus in Veterinary Practice 1.1 Background Feline Calicivirus (FCV) remains one of the most clinically significant viral pathogens affecting domestic cats, particularly as a leading cause of upper respiratory tract infections (URTIs). Its impact is amplified by the virus’s intrinsic biological properties: FCV exhibits high genetic variability, a remarkable ability to persist in chronically infected carriers, and environmental stability that enables sustained circulation within shelters, colonies, and multi-cat households. These features contribute not only to recurrent outbreaks but also to efficient viral maintenance within feline populations worldwide. From a virological standpoint, FCV is a small, non-enveloped, icosahedral virus, measuring approximately 30–40 nm in diameter and harboring a single-stranded positive-sense RNA genome of roughly 7.7 kb. Its use of junctional adhesion molecule-1 (JAM-1) as a cellular receptor facilitates viral entry and dissemination. Infection typically begins following exposure through the nasal, oral, or conjunctival routes, with the oropharynx serving as the primary site of replication. Viral amplification here drives epithelial necrosis, leading to the characteristic oral ulcerations most commonly identified along the margins of the tongue. Given the combination of widespread prevalence, substantial morbidity, and occasional highly virulent systemic outbreaks, a consolidated review of FCV’s structure, replication biology, epidemiology and clinical behavior remains essential for contemporary veterinary practice. 1.2 Objectives This article aims to synthesize current evidence from molecular virology, clinical epidemiology and field management to guide veterinary professionals, shelter medicine practitioners and cattery managers. Summarize evidence-based findings on FCV structure, replication and pathogenesis.A molecular understanding of FCV underpins rational approaches to diagnosis, prevention and therapeutic intervention. Particular focus is placed on capsid architecture, genomic organization, antigenic variability and the early host–virus interactions that shape clinical outcomes. Provide veterinarians and cattery managers with practical prevention and care strategies.In high-density environments, controlling FCV transmission requires a layered approach that combines:• vaccination programs,• minimizing population stress and overcrowding,• rigorous hygiene and environmental disinfection, and• timely clinical management of affected cats. Although vaccination remains central to disease mitigation, it typically does not prevent infection or viral shedding, and breakthrough infections continue to occur. Effective clinical management therefore relies heavily on supportive measures such as intravenous fluid therapy for dehydration, nonsteroidal anti-inflammatory drugs for pyrexia and oral pain, and targeted antibiotics for secondary bacterial complications. Highlight key clinical risks, including oral ulceration, FCV-associated lameness and virulent systemic disease (VSD). Oral Ulceration:A hallmark of classical FCV infection, especially in kittens, appearing after an incubation period of 2–10 days. It is frequently accompanied by sneezing and serous nasal discharge. FCV-Associated Lameness:Characterized by acute synovitis with joint effusion and synovial membrane thickening. This syndrome may arise days to weeks after respiratory signs or following vaccination. • Virulent Systemic Disease (VSD):A rare, highly pathogenic phenotype with reported mortality rates up to 67 percent. VSD is marked by systemic inflammatory response syndrome, disseminated coagulopathy and multi-organ failure. Clinically, affected cats exhibit severe URTI signs followed by cutaneous ulcerations, alopecia of the extremities, broncho-interstitial pneumonia and necrosis of major organs including the liver, spleen and pancreas. Management requires intensive supportive care, often incorporating corticosteroids and interferon. 2. Viral Structure and Molecular Biology Feline Calicivirus (FCV) belongs to the Caliciviridae family, a group of small, non-enveloped RNA viruses characterized by compact genomes and efficient replication strategies. The structural and molecular features of FCV underpin its clinical behavior, including its ability to persist, diversify, and evade immune surveillance in feline populations. 2.1 Virion Architecture FCV is a non-enveloped, icosahedral virus measuring 30–40 nm in diameter. Its capsid is considered “naked”, reflecting the absence of a lipid envelope. The virion is composed of a single capsid protein, with a precursor mass of 65–66 kDa, which is later processed into the major structural protein VP1. This protein forms the characteristic icosahedral shell that encases the viral RNA genome. 2.2 Genome Organization The genome of FCV consists of a single-stranded, positive-sense RNA molecule of approximately 7.7 kb, organized into three Open Reading Frames (ORFs): ORF1 – Non-structural Polyprotein Encodes a 200 kDa polyprotein that undergoes proteolytic cleavage to produce six mature non-structural proteins, essential for RNA replication and virion assembly. ORF2 – Capsid Precursor (preVP1) Encodes a 73 kDa capsid precursor (preVP1).• Undergoes rapid cleavage during maturation to yield the 60 kDa VP1 capsid protein.• Subdivided into regions A–F; among these, the E-region determines antigenicity and contributes to formation of the P2 subdomain, a key external protrusion involved in receptor interaction and immune recognition. ORF3 – Minor Structural Protein VP2 Encodes VP2, a 12 kDa protein (106 amino acids) that, although less abundant, is essential for producing infectious virions and supports VP1 stability during assembly. https://share.google/unsumbG6JXsez5x1R 2.3 Replication Cycle FCV replication proceeds through the synthesis of: A 7.7 kb genomic RNA (positive-sense)• A 2.4 kb subgenomic RNA, which serves as the template for capsid protein translation Viral entry is mediated by junctional adhesion molecule-1 (JAM-1), identified as the functional receptor during in vitro studies. Once inside the host cell, FCV induces a characteristic cytopathic effect (CPE)—notably cell rounding and membrane blebbing. A central mechanism of viral dominance is the shut-off of host protein synthesis, accomplished through cleavage of eIF4G, a critical eukaryotic initiation factor. This redirection enables preferential translation of viral RNA and efficient progeny production. 3. Epidemiology 3.1 Global Distribution Feline Calicivirus (FCV) was first isolated from the gastrointestinal tract of cats in New Zealand (Fastier, 1957). Since that initial discovery, FCV has been recognized as a globally widespread pathogen, circulating in domestic and free-roaming feline populations across continents. Its prevalence is particularly high in high-density environments, such as multi-cat households, shelters, and breeding colonies. Studies consistently report 25–40 percent infection rates among cats in colonies and shelters (Wardley et al. 1974, Bannasch & Foley 2005). The combination of environmental persistence, antigenic diversity, and efficient cat-to-cat transmission ensures FCV remains an endemic viral pathogen in most feline communities worldwide. 3.2 Transmission and Persistence FCV transmission occurs predominantly through oral, nasal, or conjunctival exposure. Direct contact with secretions, as well as indirect exposure through contaminated fomites, facilitates rapid spread—particularly in
Canine blood-typing
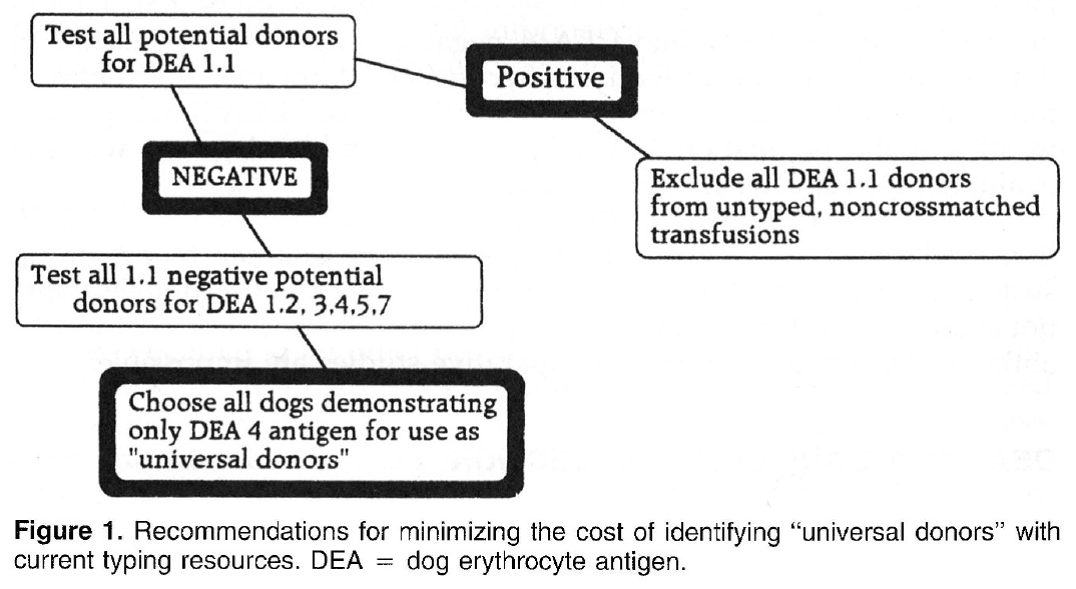
Canine blood-typing Andy Pachikerl, Ph.D Introduction: Dog erythrocyte antigens are responsible for initiating approximately 70% to 80% of immune-mediated transfusion reactions in the dog. As with other species, the red blood cell antigens found in the dog have various immunogenicities. In health, these antigens are participants in cell recognition-self versus nonself. In disease, they may serve as antigens for antibody or markers in disease occurrence. Little is known about their biochemical properties. Currently their description is reliant on polyclonal antibody serology. This reliance has limited the progress of transfusion practice in the dog. Historically, the study of canine blood groups and their importance in transfusion began in the 1600s through a physician, Richard Lower. He is credited with the first canine-to-canine transfusion. The efforts of doctors Lower and Denis in heterologous transfusion using lamb, dog, and human subjects introduced the basic transfusion premise “like transfuses like.” In 1910, Von Dungem and Hirszfeld documented the presence of four hemolysins and agglutinins based on canine alloimmunization (Swisher & Young, 1961). Further work by Ottenburg, Kaliski, and Friedman in 1913 confirmed these findings. From 1937 to 1949, Wright, Whipple, and Eyquem further defined the presence of six canine blood groups (Colling & Saison, 1980). However, not until 1961 was the importance of these antigens in transfusion and disease investigated by Swisher, Young, and Trabold (Swisher, et al., 1962). To date, the work submitted by Swisher and Young remains the most current published information of the importance of canine blood groups in transfusion (Swisher, 1954; Swisher, et al., 1962; Young, et al., 1951). Additional blood groups have been identified by Rubenstein (1968), Suzuki/5 Colling and Saison (Colling & Saison, 1980; Colling & Saison, 1980), and Symons and BelLl9 (Symons & Bell, 1992). Of this latter group, only the antigen first noted by Rubenstein (Colling & Saison, 1980) was evaluated in regard to transfusion significance by Bull (Bull, 1976). The importance of canine blood groups in veterinary transfusion medicine is based on three factors: the incidence of the antigen in the dog population, the incidence of naturally occurring antibody within the population, and the effect of the antibody against the antigen in vivo. Current blood typing schemes identify six erythrocyte antigens with possible importance. The dog erythrocyte system Blood groups are defined by glycolipids and glycoproteins on the surface of the red blood cell membrane. Current blood typing schemes identify six dog erythrocyte antigens (DEAs): 1.1, 1.2, 3, 4, 5, and 7 (Table 1). Blood groups are independently inherited. Simple Mendelian laws of dominance govern their inheritance. These antigens are defined by using polyclonal antibodies generated through canine alloimmunization. Polyclonal antibody recognition may be dependent on multiple recognition sites to define the “antigens” currently accepted. Biochemically, little is known about the DEA system. Table 1. Dog erythrocyte antigens established as international standards: classification, occurrence, and significance This blood group system has been defined with multiple alleles. They include the antigens 1.1, 1.2, 1.3, and a null type. An individual dog may show only one of the four phenotypes. Family studies suggest a Mendelian type of autosomal dominance. Table 2 describes the current phenotypic and genotypic information on this blood group system. 1.1- and 1.2-positive dogs have been studied for transfusion significance. Naturally occurring antibody to these alleles has not yet been found. Therefore, first-time transfusion reactions do not occur. However, if a negative dog is exposed to 1.1- positive erythrocytes, a strong hemolysin can result. On second exposure, an immune-mediated hemolytic transfusion reaction results causing removal of transfused cells in less than 12 hours. Hemoglobinuria and hyperbilirubinemia frequently occur. In addition to uncross matched, untyped transfusion, pregnancy can cause production of antibody against DEA 1.1 25% of the time. For these reasons 1.1-positive dogs are excluded as transfusion donors. 1.2-positive dogs can cause a problem as both the transfusion donor and recipient. A previously sensitized negative type dog undergoes permanent red blood cell removal and loss 12 to 24 hours after the administration of 1.2-positive red blood cells. Thus, 1.2-positive dogs are poor erythrocyte donors. If a 1.2-positive dog is sensitized with DEA 1.1 red blood cells, it will produce a potent anti-DEA 1.1 antibody. Administration of DEA 1.1 red blood cells to a sensitized 1.2 dog results in an immediate hemolytic transfusion reaction. Therefore, 1.2-positive dogs are at risk after sensitization for immediate transfusion. 1.3-positive dogs have not been evaluated for transfusion significance. Future study is limited because of the unavailability of typing sera for DEA 1.3. DEA 7 This red blood cell antigen is the most controversial among the six antigens discussed. Published reports of naturally occurring antibody to this antigen suggest that this antibody has a natural prevalence as high as 50% in DEA 7-negative dogs. Recent reports by Giger fail to support the presence of naturally occurring anti-DEA 7. Observations by the author suggest that naturally occurring antibody does exist in 20% to 50% of all DEA 7-negative dogs. However, the naturally occurring antibody is quite weak, rarely producing a titter greater than 1:8. In the presence of naturally occurring antibody, as in the cat, immunemediated transfusion reaction can occur during a first transfusion. Sensitized DEA 7-negative dogs, when transfused with DEA 7-positive erythrocytes show a delayed transfusion reaction. Hemolysis does not occur; however, an irreversible sequestration and loss of red blood cells occurs in 72 hours. This type of delayed transfusion reaction is only significant if the regenerative ability of the transfusion recipient is compromised. Because of the presence of naturally occurring antibody in the DEA 7-negative population and because of the delayed loss of erythrocytes in sensitized dogs, DEA 7-positive dogs are not recommended as donors. DEA3 This antigen has not been considered significant because of its low incidence in the dog population of the United States. However, recent evaluation of DEA type by breed suggests that it may be more important. Only 6% of the general population has DEA 3-positive cells. Yet 23% of the Greyhounds typed from 1990 to
Case study: Cerebral toxoplasmosis in a cat with feline leukemia and feline infectious peritonitis viral infections
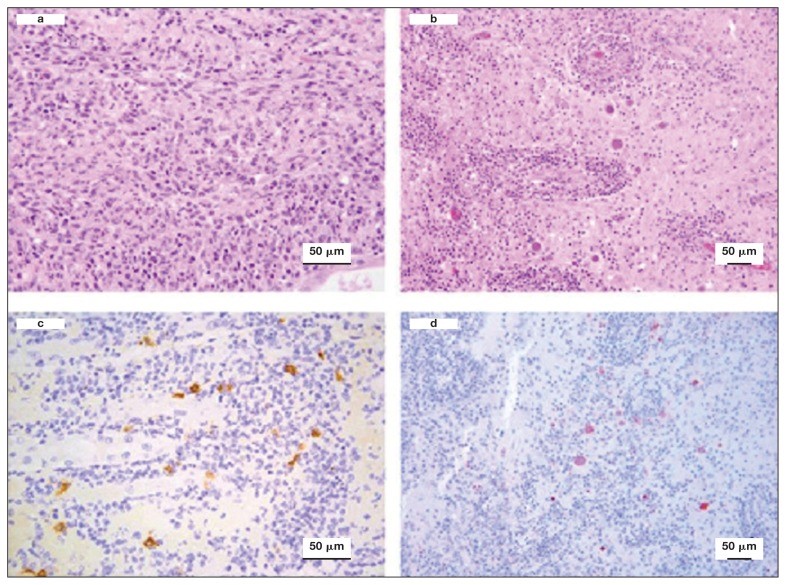
Case study: Cerebral toxoplasmosis in a cat with feline leukemia and feline infectious peritonitis viral infections Robert Lo, Ph.D, D.V.M A diarrheic young cat died because of severe multifocal meningoencephalitis caused by Toxoplasma gondii. Protozoan cysts and tachyzoites in the brain were confirmed by immunohistochemical staining. Coinfection of feline leukemia virus (FeLV) and feline infectious peritonitis (FIP) might be the possible contributors to the clinical, fatal outcome. Original paper: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6049326/ Histological lesions and immunohistochemistry (IHC) of the brain and kidney of a 6-month-old cat that was diagnosed with cerebral toxoplasmosis following postmortem examination. a — Kidney showing chronic pyogranulomatous nephritis. Hematoxylin and eosin (H&E), 40×. b — Brain showing perivascular cuffing of lymphocytes and plasma cells with multifocal vasculitis. Many oval protozoan cysts having a thin wall and containing basophilic bradyzoites were seen near to the vascular structures. H&E, 20×. c — Kidney stained by IHC with feline coronavirus antibodies showing multifocal positive reaction in the cytoplasm of macrophages, 40×. d — Brain stained by IHC with anti-Toxoplasma gondii antibodies and showing several positively stained protozoan cysts and tachyzoites, 20×.
The Feline Herpesvirus: An Overview
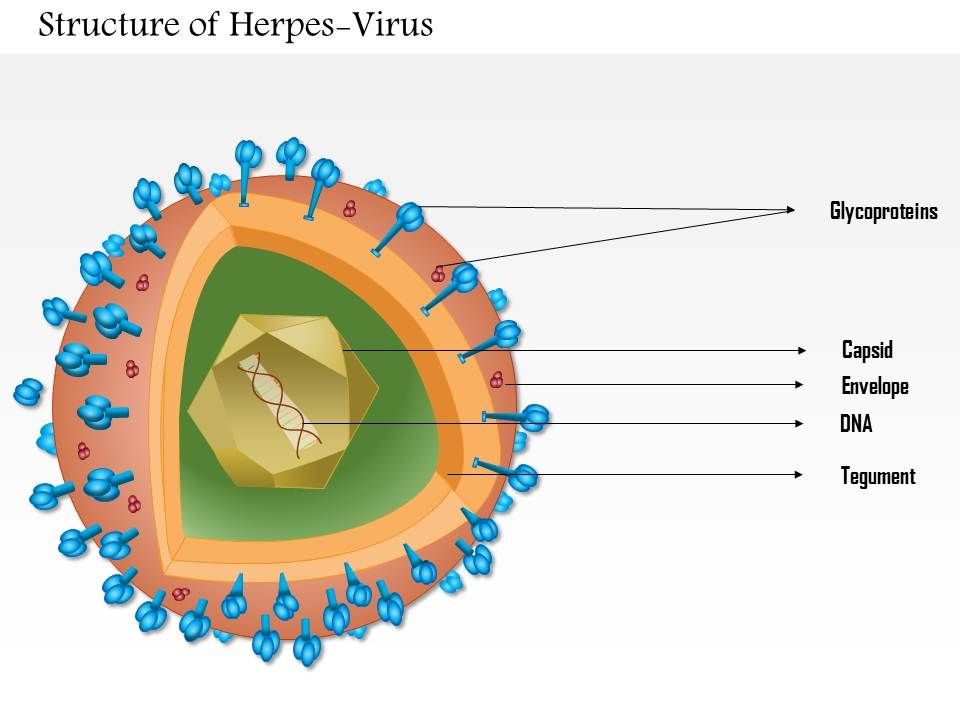
The Feline Herpesvirus: An Overview Maigan Espinili Maruquin The feline herpesvirus infection is common and recurring ocular disease is common (Stiles 2000). It is the most studied infectious cause of ocular surface disease in cats (Andrew 2001). Developing latent infections may recrudesce at later points in life of an infected cat (Stiles 2000). I. Structure and Replication Fig. 01. Structure of Herpes Virus. https://www.slideteam.net/0814-herpes-virus-medical-images-for-powerpoint.html The feline herpesvirus (FHV 1) causes feline viral rhinotracheitis (FVR) (Gaskell and Wardley 1978, Gaskell, Dawson et al. 2007, Henzel, Brum et al. 2012). This virus is double-stranded DNA with a glycoprotein-lipid envelope and is a member of the Varicellovirus genus in the Alphaherpesvirinae subfamily (Gaskell, Dawson et al. 2007). This virus was also found out to be relatively fragile in the external environment and is highly susceptible to the effects of common disinfectants (Scott 1980, Eleraky, Potgieter et al. 2002, Gaskell, Dawson et al. 2007). The FHV- 1 has short replication cycle, rapid cell-to-cell spread, has tendency to induce cell lysis, and displays persistence in sensory ganglia of their host (Gould 2011). It replicates in epithelial cells of both the conjunctiva and upper respiratory tract, and in neurons. The neuronal infection can lead to a lifelong latency after the primary infection (Thiry, Addie et al. 2009). For 18 hours, it can survive in damp environment, less in dry conditions and is also recorded to be relatively unstable as an aerosol (Povey and Johnson 1970, Donaldson and Ferris 1976, Stiles 2000, Gaskell, Dawson et al. 2007, Gould 2011). II. Infection and Epidemiology There are only three main genotype groups recognized for FHV-1 strains with very little genomic variations (Gould 2011). The virus sheds in ocular, nasal, and oral secretions with large transmission by direct contact with an infected cat. Although one of the most important sources of virus are the acutely infected cats, susceptible cats may also be infected by latently infected carrier cats (Gaskell and Povey 1982, Gaskell, Dawson et al. 2007). On the other hand, the environment may not be a primary source of transmission but catteries may cause indirect transmission through contaminated housing, feeding and cleaning utensils, and personnel (Gaskell, Dawson et al. 2007, Thiry, Addie et al. 2009). Latently infected cats may also transmit FHV to their kittens due to the parturition and lactation inducing stress that may lead to viral reactivation and shedding, making kittens susceptible to the virus, depending on the level of maternally derived antibodies (MDA) they possess. High levels of MDA protects kittens against the disease and may develop subclinical infection leading to latency while insufficient MDA may lead to clinical signs (Gaskell and Povey 1982, Thiry, Addie et al. 2009). Recovered cats become latently infected carriers and reactivation happens particularly after periods of stress (Gaskell, Dawson et al. 2007). However, it doesn’t shed immediately after the stress. It undergoes lag phase of 4–11 days, precedes the shedding from 1–13 days (Gaskell and Povey 1973, Gaskell and Povey 1977, Gaskell, Dawson et al. 2007). Further, risk factors associated with FeHV-1 shedding includes contact with other cats, the presence of upper respiratory disease, younger cats, poor hygiene, and larger households (Sykes, Anderson et al. 1999, Binns, Dawson et al. 2000, Helps, Lait et al. 2005, Gaskell, Dawson et al. 2007). III. Pathogenesis Infection routes include nasal, oral, and conjunctival mucous membranes and is primarily shed in secretions for 1–3 weeks following infection (Andrew 2001, Gaskell, Dawson et al. 2007). In pregnant queens, vaginitis was caused by intravaginal instillation virus and causes congenitally infected kittens while intravenous inoculation leads to transplacental infection and abortion (Bittle and Peckham 1971, Hoover and Griesemer 1971, Gaskell, Dawson et al. 2007). After 1 to 2 exposure of naive animals to FHV-1, the viral replication happens and epithelial cell necrosis occur in the nasal turbinates, nasopharynx and conjunctival mucosa (Gaskell & Dawson 1998). Lytic infection of the nasal epithelium with spread to the conjunctivas, pharynx, trachea, bronchi and bronchioles occurs and lesions characterized by multifocal epithelial necrosis with neutrophil infiltration and inflammation are also observed. Moreover, neonates or hypothermic kittens display transient viraemia associated with mononuclear cells (Gaskell, Dawson et al. 2007, Thiry, Addie et al. 2009). It has been recorded that almost all infected cats become lifelong carriers. During the latency period, virus was spread along the sensory nerves and neurons with viral genome doesn’t replicate. Whereas, reactivating stressors include lactation and moving into a new environment (Gaskell and Povey 1977, Gaskell and Povey 1982, Pedersen, Sato et al. 2004, Thiry, Addie et al. 2009). Lesions may be developed upon viral reactivation in adult cats and ‘recrudescence’ disease may also be a consequence (Thiry, Addie et al. 2009). As high as 70% mortality rates was reported for infected kittens (Povey 1990). Although MDA may persist for 2 to 10 weeks, this may not protect cats from subclinical infection (Gaskell & Dawson 1998)(Andrew 2001). IV. Clinical Signs Generally, FHV- infected cats display acute upper respiratory and ocular disease with usually 2 to 6 days incubation period, or may be longer (Gaskell and Povey 1979, Stiles 2000, Gaskell, Dawson et al. 2007) with depression, fever, lethargy, inappetence, pyrexia, sneezing, coughing, nasal discharge, and conjunctivitis with ocular discharge depending on the viral exposure and individual susceptibility (Hoover, Rohovsky et al. 1970, Crandell 1973, Stiles 2000, Gaskell, Dawson et al. 2007, Thiry, Addie et al. 2009) (Gaskell R.M., Dawson S, 1994). Also, excessive salivation with drooling may also be observed during the initial clinical signs of the disease (Gaskell, Dawson et al. 2007).Once the virus reaches the lungs, pneumonia may kill the infected kittens (Stiles 2000, Thiry, Addie et al. 2009) (Gaskell R, et al. 2006). The primary FHV- 1 infection with secondary bacterial infection leads in conjunctivitis sometimes with severe hyperemia and chemosis. The conjunctivitis is manifested as hyperaemia or redness with serous discharge, progressing to mucopurulent ocular discharge whereas, chemosis is swelling or oedema of the conjunctiva which may occur to
Symmetric dimethylarginine (SDMA)
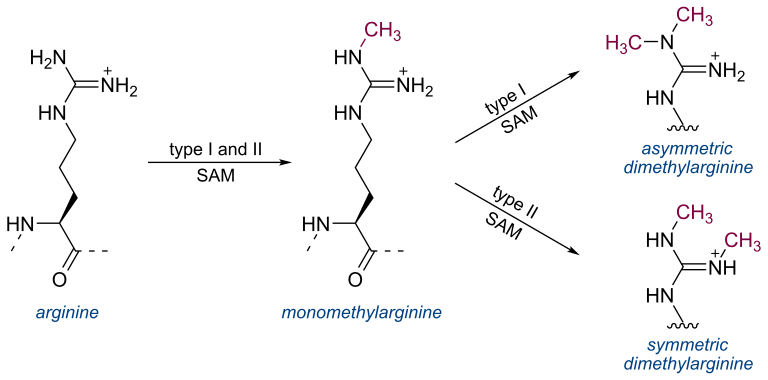
Symmetric dimethylarginine (SDMA) Andy Pachikerl, Ph.D Introduction For over a millennium and a few centuries, urinalysis has given leads to medical diagnoses. It was until the repetitive use of clinical chemistry approximately 50 – 60 years that these data of renal biomarkers became commonplace in human and veterinary medicine. From here onwards, both an improved understanding of the renal system and ability to diagnose renal disease was updated. In the past, renal biomarkers have focused on kidney function testing, and this is the basis for current conventional test in blood (serum creatine [sCr], urea or urea nitrogen [UN] as endogenous indicators of glomerular filtration rate [GFR]). Recently, we are becoming more aware of the need to identify renal disease at an early stage when therapeutic options are most effective. Both sCr and UN both play vital role in diagnosis of kidney disease, their limitations create poor confidence for their use as early indicators for disease. New markers of renal function try to overcome these limitations. Additionally, there are now many urinary markers that can detect kidney damage and help localize that damage to the compartment of the kidney that is affected. Endogenous markers of GFR Creatine The most common endogenous marker for estimating GFR is serum creatinine and its metabolism. Measurement and diagnostic significance in dogs have previously been reviewed (Braun, et al., 2003). Recent reviews, however, suggested factors that can either enhance or limit the clinical use of sCr to optimize diagnostician and clinical pathologist to interpret the data of this conventional test. Particularly, accurate interpretation of published data, population-specific reference intervals, trending of sCr and consideration of muscle mass influence and analytic variability are all needed to best interpret sCr in dogs and cats. Of note, although creatinine is referred to as sCr throughout this manuscript, creatinine is also commonly measured in plasma. Nephron mass vs nephron function. It is generally accepted that 75% of nephron mass must be lost before sCr increases above the reference limit (Braun, et al., 2003). The original source for this statement likely originates from partial nephrectomy studies in dogs. However, it is often mistaken for 75% loss of renal function vs mass. In partial nephrectomy studies, ¾ loss of renal mass related to about 50–60% or 35–45% reduction in renal function based on inulin clearance one month or 13 months post-surgery, respectively (Brown, et al., 1990; Bovee, et al., 1979). The much lower decrease in function as compared with the percentage of nephron loss is due to compensatory changes in remaining nephrons (ie, compensatory renal hypertrophy) (Brown, et al., 1990; Bricker, et al., 1964). Furthermore, using an age- and breed-specific reference limit (sCr ≥ 106 mmol/L or 1.2 mg/dL) along with frequent monitoring, adolescent dogs with rapidly progressive kidney disease due to X-linked hereditary nephropathy (XLHN) demonstrated increased sCr after GFR had decreased an average of 48% (range 39–68%).8 Based on these studies, sCr can be more sensitive for detecting decreased renal function than has been historically assumed. Value of population-specific reference intervals. While sCr is not as poorly sensitive as generally believed, its inability to regularly detect < 50% decline in kidney function at least partly stems from reference intervals that are overly wide for patients with low baseline sCr. Since current methodologies are highly specific for creatinine, the wide reference intervals largely stem from biologic differences in sCr among individuals. Serum creatinine has relatively high individuality in dogs and cats (Baral, et al., 2014; Ruaux, et al., 2011), meaning that variability between individuals is much higher than the variability observed within a single animal. Serum creatinine is influenced by age (Rosset, et al., 2012; Rørtveit, et al., 2015) and particularly by breed in dogs (Misbach, et al., 2014; Zaldívar-López, et al., 2011) and, to a lesser extent, in cats.20 It might also be influenced by sex and the veterinary clinic evaluating the patient.21 Therefore, sCr would benefit from age- and breed-specific reference intervals, ideally (although not practically) for every individual instrument and laboratory. Trending of serum creatinine. Small increases in sCr even within the reference interval can reflect significant decreases in GFR in an individual patient8, particularly since variation in sCr within an individual healthy dog or cat is minimal over weeks to months and even years (Braun, et al., 2003; Baral, et al., 2014; Ruaux, et al., 2011). In fact, the critical difference or reference change value for detecting a significant increase or decrease in sCr is only 23–27 lM/L (0.3 mg/dL) in clinically healthy dogs10, and 17% (corresponding to similar absolute values as in dogs) in clinically healthy cats (Braun, et al., 2003). Thus, the sensitivity of sCr for detecting early kidney disease can be improved by evaluating serial fasted sCr measurements in an individual animal (trending) to look for increases that likely reflect worsening renal function. This concept of detecting small but clinically relevant increases in sCr is actively being adopted in cases of acute kidney injury (AKI), illustrated by the International Renal Interest Society (IRIS) Grading of AKI. In this grading scheme, an increase in sCr ≥ 26 lmol/L (0.3 mg/dL) within a 48- hour period is a criterion for identifying Grade I and Grade II AKI (www.IRIS-Kidney.com). Furthermore, in adolescent dogs with XLHN, trending of sCr detected an average of 27% (range 5–49%) decrease in GFR (Nabity, et al., 2015). Despite heightened awareness of small, but clinically relevant increases in sCr over a short time frame, more recognition is needed with slowly progressive CKD, in which small increases might occur over many months or years. Analytic challenges. Finally, sCr is plagued by inconsistencies in its measurement between instruments and laboratories, which can result in markedly different results. While most reference laboratory instruments have excellent precision and provide results of similar magnitude among instruments (Ulleberg, et al., 2011), recent studies illustrate the high imprecision and bias possible with some instruments and among different laboratories (Ulleberg, et al., 2011; Braun, et al., 2008). In normal to mildly azotaemia samples,
