Case study: Report of the first clinical case of intestinal trichomoniasis caused by Tritrichomonas foetus in a cat with chronic diarrhoea in Brazil
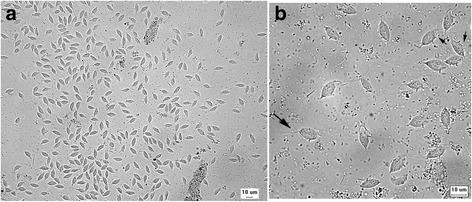
Case study: Report of the first clinical case of intestinal trichomoniasis caused by Tritrichomonas foetus in a cat with chronic diarrhoea in Brazil Robert Lo, Ph.D, D.V.M A seven-month-old, entire male domestic shorthair kitten was presented to the Veterinary Hospital of the School of Veterinary Medicine – University of São Paulo, Brazil. The cat showed a six-month history of persistent large intestinal diarrhoea, faecal incontinence, prostration, apathy and weight loss. P Protozoan parasites were observed under microscope using fresh fecal sample obtained via colon flush. Infection of Tritrichomonas foetus was confirmed by PCR and DNA sequencing. After treatment with ronidazole (30 mg/kg, PO q24h for 14 days), the cat showed resolution of clinical signs. This is the first clinical case of T. foetus infection in a chronic diarrheic cat in Brazil and South America. Original paper: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5392982/ Fig. 1 Tritrichomonas foetus in cat feces. a Numerous pyriform trophozoites. b The three free anterior flagella (large arrow) and the undulating membrane (small arrows) can be visualised in some trophozoites. Fresh preparation in saline 0.85%.
Feline Pancreatic Lipase
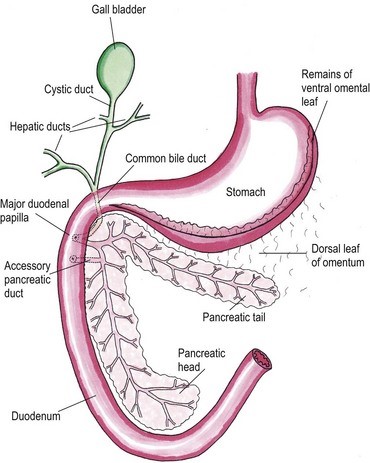
Feline Pancreatic Lipase LIN, WEN-YANG (WESLEY), Ph.D Feline Pancreatic Lipase is a powerful diagnosing biomarker for feline pancreatitis, whereas serum amylase and serum lipase are usually used in diagnosing pancreatitis for canine, are not effectively in detecting pancreatitis for cats. Anatomical physiology function of pancreas in cats The feline pancreas is a digestive glandular organ, which presents a long v-shaped strip of configuration locating at abdomen between stomach and duodenum. The tail part of feline pancreas rests toward the dorsal extremity of the spleen and connect to mesocolon with omentum (Figure 1). Figure 1. Anatomic view of the pancreas and its surrounding tissues https://veteriankey.com/pancreas-4/ The normal pancreas colored on pale pink and perform both functions of endocrine and exocrine. The endocrine portion contains small clusters of pancreatic α- and β-cells within Langerhans (approximately 2% of the gland’s weight) that majorly generates two hormones proteins: glucagon and insulin. Glucagon works to elevate the level of blood sugar, whereas insulin would diminish excess circulating blood sugar. Both of glucagon and insulin would take part in regulating homeostasis of glycemic level. Acinar and ductal cells are major members in the exocrine portion of pancreas. Acinar cells aggregate around the terminal pancreatic ductules to secrete digestive enzymes include trypsinogen, chymotrypsinogen, proelastase, procarboxypeptidase, ribonucleases, deoxyribonucleases, phospholipase A2, carboxylesterase amylase and lipase. Amylase and lipase are contributing to hydrolyze carbohydrates and fat. Others may play roles as proteases to cleave polypeptide chains. Feline’s ductal cell generates antibacterial proteins to protect small intestinal from bacterial infection. Moreover, ductal cell can also produce bicarbonate and water for neutralizing pH in the duodenum. Intrinsic factor such as vitamin B12 would be made from ductal cell too. The pancreas is the only generating organ of intrinsic factor for feline, whereas dogs could produce intrinsic factor from pancreas and stomach. All pancreatic enzymes would secret into small intestine for digesting fats, proteins and carbohydrates. Furthermore, the abnormal seep of exocrine enzymes to pancreas and its surrounding organs could cause pancreatic inflammation known as pancreatitis. The highest occurrence of feline pancreatic diseases is exocrine pancreatic insufficiency (EPI) and pancreatitis. Pancreatitis in the cat Feline pancreatitis is classified as acute (temporary morphological changes) and chronic (permanent morphological change) type according to the condition of histopathologic changes after treatment. Acute necrotizing pancreatitis (ANP) and acute suppurative pancreatitis are two of the most common types of acute feline pancreatitis, whereas chronic non-suppurative pancreatitis (CP) and pancreatic atrophy are chronic conditions. Common acute pancreatitis in feline would show anemia, leukocytosis, hypokalemia, hypocalcemia, hyperglycemia, elevation of ALT, ALP, total bilirubin, cholesterol and decreasing level of albumin. Acute necrotizing pancreatitis (ANP) usually present pancreatic acinar cell necrosis, peripancreatic fat necrosis followed inflammation, hemorrhage, mineralization and fibrosis. Despite of its idiopathic character, several diseases have considered to be related with development of ANP including concurrent biliary tract disease, ischemia, pancreatic ductal obstruction, toxoplasmosis, feline Herpes virus infections, feline infectious peritonitis, pancreatic fluke infestations (Eurytrema procyonis, Amphimerus pseudofelinus), trauma, organophosphate poisoning and hepatic lipidosis. General anesthesia induced hypotension or surgical venous outflow occlusion would decrease pancreatic blood flow and cause ANP. Acute suppurative pancreatitis is less common than ANP in feline and neutrophilic inflammation would happen with it. Besides, the continuous and progressive inflammatory process of the pancreas would lead to chronic non-suppurative pancreatitis (CP) in which lymphocytic inflammation, fibrosis, and acinar atrophy are the major features. The end stage of CP in most feline cases would usually result in pancreatic atrophy, which may or may not influence the endocrine portion of the gland. Felines who suffer pancreatic atrophy would occur cobalamin and fat-soluble vitamin malabsorption, severe maldigestion, acid injury in duodenal mucosa, and bacterial proliferation in the gut due to exocrine pancreatic insufficiency. Since various types of pancreatitis such as acute and chronic pancreatitis, pancreatic abscess, pancreatic cyst/pseudocyst, exocrine pancreatic insufficiency, and neoplasia share overlapping symptoms, thus histopathology cab be used to discern different conditions. Symptoms of feline pancreatitis Symptoms of feline pancreatitis would occur lethargy, anorexia, dehydration, hypothermia, vomiting, weight loss, Jaundice, cholangiohepatitis, hepatic lipidosis, biliary obstructions, cranial abdominal masses and cranial abdominal discomfort. Besides, hepatic and intestinal disease would concurrent. I. Acute feline pancreatitis: Vomiting, poor appetite, poor activity, diarrhea, abdominal pain, drooling, fever, collapse. II. Chronic feline pancreatitis: Frequent vomiting, poor appetite, listless, frequent diarrhea, abdominal pain, drooling, fever, collapse, hypothermia, breathing too fast or too slow, fast heartbeat. Possible Causes of feline pancreatitis Scientists presumed that premature trypsin activate of digestive zymogens in pancreatic acinar cells would cause pancreatic autodigestion, acinar cell necrosis, hemorrhage, and fat necrosis, saponification, mast cell degranulation, leukocyte chemotaxis, platelet aggregation, vasodilation, surfactant degradation within the lungs and initiation of disseminated intravascular coagulation (DIC) for worse cases. Diagnosis and general considerations Since symptoms of feline pancreatitis were similar to common flu, vets probably can’t judge it correctly at the early stage without proper diagnostic tools. It’s not an easy task to diagnose pancreatic disease within cats. Single diagnostic method is not recommended. The diagnosis of feline pancreatitis relied on combinational diagnostic tools include historical data, physical examination results, laboratory, and Bio-imaging. Especially, histopathology plays the role as the definitive conclusion for feline pancreatitis. Middle-aged felines are susceptible to pancreatitis; whereas, older felines (mean 12.8 years, range 4–20 years) are susceptible to neoplasia, cyst/pseudocysts. Diagnostic imaging Radiography, computed tomography (CT) and ultrasonography were effective imaging tools applied in diagnosing feline pancreatitis. Among all imaging tools, ultrasonography is the most reliable imaging modality for the diagnosis of feline pancreatic diseases, which can help identify soft tissue masses, cysts/pseudocysts, abscesses, or neoplasia and lesions of feline pancreatitis. Clinicopathologic tests The general clinicopathologic test for feline pancreatitis include complete blood cell count, serum bilirubin, cholesterol, glucose, total protein, albumin, and serum activity of liver enzymes (serum alanine aminotransferase and alkaline phosphatase), calcium, urea, creatinine, and potassium. However, serum lipase and amylase activities can’t be diagnostic indicator for feline pancreatic disease. Nevertheless, feline trypsin-like immunoreactivity (fTLI) and
Feline Alpha-1-acid glycoprotein (AGP)
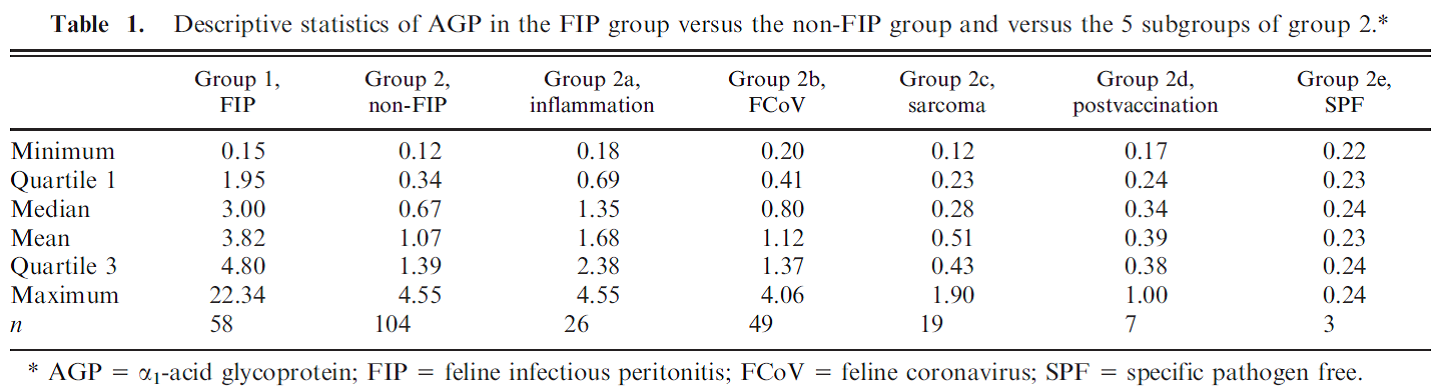
Feline Alpha-1-acid glycoprotein (AGP) Andy Pachikerl, Ph.D Introduction Alpha-1-acid glycoprotein (AGP) surges in cats’ blood when they fall in victim of feline infectious peritonitis (FIP), a lethal disease caused by feline coronavirus (FCoV). The diagnosis of feline infectious peritonitis (FIP) is often tough and not very viable at times. The clinical suspicion of FIP might be supported by the detection of effusions if any are present (Hartmann, et al., 2003; Paltrinieri, Parodi, & Cammarata, In Vivo Diagnosis of Feline Infectious Peritonitis by Comparison of Protein Content, Cytology, and Direct Immunofluorescence Test on Peritoneal and Pleural Effusions, 1999). The only way to conclusively test FIP to be positive is through histology followed by immunohistochemical or immunofluorescent detection of feline coronavirus (FCoV) within intralesional macrophages (Addie, Paltrinieri, & Pedersen, 2004; Barlough & Stoddart, 1990). Various studies suggested the implementation of biopsies for the confirmation of FIP in veterinary practices and it turns out to be quite useful with just a few downside (Alessia, Paltrinieri, Bertazzolo, Milesi, & Parodi, 2005). The application of biopsies in vivo is usually restricted due to anaesthetic risks especially via surgical biopsy and the relatively high percentage of unsuitable or falsely negative tru-cut or fine-needle aspiration biopsies (Alessia, Paltrinieri, Bertazzolo, Milesi, & Parodi, 2005). Serology and polymerase chain reaction techniques are not suitable for FIP diagnosis because they do not differentiate between the widespread low pathogenic FCoVs and the mutant pathogenic FCoV strains (Addie, Paltrinieri, & Pedersen, 2004; Herrewegh, et al., 1997). A previous study (Stoddart, Whicher, & Harbour, 1988) reported high levels of a1-acid glycoprotein (AGP) in cats with experimentally induced FIP. This finding was confirmed by another study, (Duthie, Eckersall, Addie, Lawrence, & Jarrett, 1997) which proposed the possible use of serum AGP as a diagnostic tool for FIP. Serum AGP is now widely used in diagnostic profiles for FIP.1 However, serum AGP levels increase in inflammatory disorders other than FIP (Duthie, Eckersall, Addie, Lawrence, & Jarrett, 1997; Kajikawa, Furuta, Onishi, Tajima, & Sugii, 1999; TerWee, Lauritzen, Sabara, Dreier, & Kokjohn, 1997; TerWee, et al., 1998), neoplasia (Correa, Mauldin, Mauldin, & Mooney, 2001), and asymptomatic but FCoV-positive cats. This lack of specificity limits the diagnostic potential of serum AGP as a diagnostic test for FIP. For more information on FIP and FCoV can be found in previously published report article (link). FIP clinical diagnosis through feline AGP AGP has been used extensively, particularly in Europe, as an indicator test for FIP. AGP was found almost a decade ago to be hyposialylated in cats with FIP, but not in normal cats or in cats with other pathologies (Fabrizio, Claudia, Alessia, Vanessa, & Saverio, 2004). This study confirmed that serum AGP is a powerful discriminating marker for FIP, but only when coupled with other high risk factors (Saverio, Giordano, Tranquillo, & Guazzetti, 2007). A Bayesian approach demonstrated that, when the pre-test probability of FIP was high based on history and clinical signs, moderate serum AGP levels (1.5–2 μg / mL) could discriminate cats with FIP from others. However, only high serum AGP levels (>3 μg / mL) were highly suggestive of FIP in cats with a low pre-test probability of disease (Saverio, Giordano, Tranquillo, & Guazzetti, 2007). Giori, et al. (2011) had shown specificity and sensitivity of several tests in 12 cats, four of which have the absence of FIP via histopathology and immunohistochemistry, and eight cats with FIP confirmed via histopathology and immunohistochemistry. Results from serum protein electrophoresis, analysis of effusions, anti-feline coronavirus serology, serum AGP concentrations and histopathology were then compared with the confirmed diagnosis. No concordance was found for serology and analysis of effusions, poor concordance was noted for histopathology, fair concordance for serum electrophoresis and perfect concordance for AGP. Their study proved that immunohistochemistry is always required to confirm FIP and, if immunohistochemistry is not feasible, they concluded that histopathology is not definitive, whilst elevated AGP concentrations might support the diagnosis of FIP. However, the small numbers of cats in this study make it difficult to validate such conclusions and the earlier study of Saverio, et al. (2007) is probably a more accurate assessment of AGP testing for FIP. Like most indirect tests for FIP, the positive predictive value increases with the number of other risk factors that are present. Saverio, et al. (2007) also investigated the levels of leukocyte bound AGP in normal cats and cats with diseases including FIP by flow cytometry using an anti-feline AGP antibody. A total of 32 healthy cats (19 feline coronaviruses seropositive), 13 cats with FIP (presumably all coronavirus seropositive) and 12 cats with other diseases (six coronaviruses seropositive) were studied. The proportion of cats with AGP-positive leucocytes in each group or in cats with different intensities of inflammatory response (as measured by CBC, serum electrophoresis and serum AGP levels) was compared. AGP positive leucocytes were found in 23% of cats; most were diseased, but a small number were healthy. AGP positive leukocyte staining was associated with inflammation and not with leucocytosis per se. Staining among healthy cats was unrelated to coronavirus antibody status. Cats with FIP were more likely to have positive staining leukocytes than healthy cats, but not as likely as cats with other diseases. It was concluded that AGP positive leucocytes are present in feline blood, especially during inflammation. Staining leukocytes for AGP binding do not appear to have any value over serum AGP testing, especially when considering the potential cost and effort involved in this method. A previous study by Paltrinieri, et al. (2007) showed positive correlation between AGP and FIP cats. In their study, they used 2 different groups of cats with FIP or non-FIP along with others contracted with other diseases such as FCoV. Their schematic experiment is as follows: Group 1. FIP group. This group was composed of 58 cats that had clinical signs and laboratory findings confirmatory of effusive FIP (n 5 53) or dry FIP (n 5 5). Haematology and serum biochemistry in these cats revealed nonregenerative anaemia, neutrophilia, lymphopenia, increased total
Understanding FPV and its Threat to Our Cats
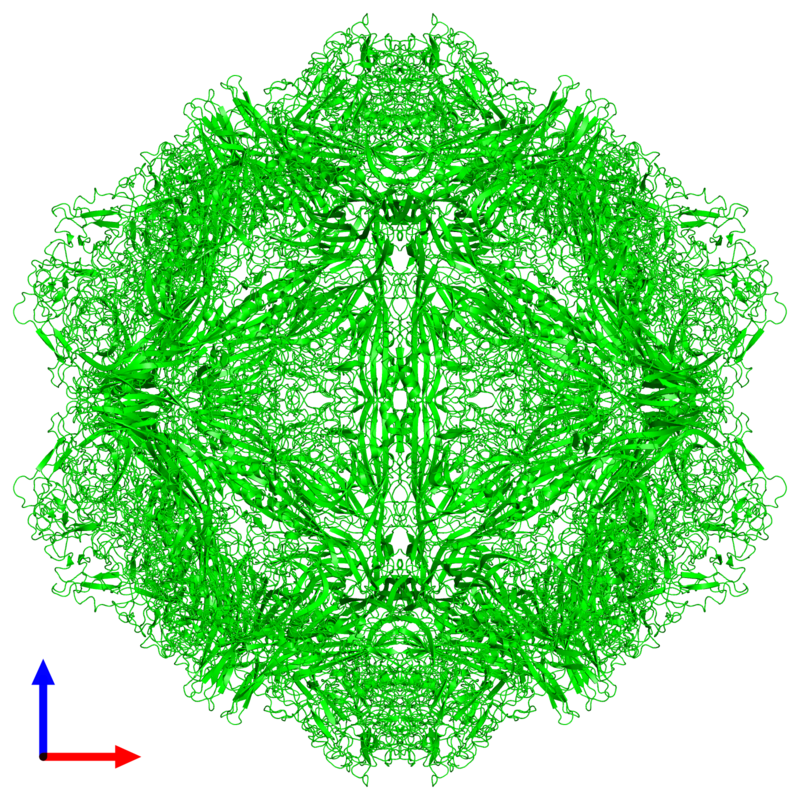
Understanding FPV and its Threat to Our Cats Maigan Espinili Maruquin The Feline Panleukopenia (FPL) is an important disease in cats. It is highly contagious and is often fatal to cats (Van Brussel, Carrai et al. 2019). This is caused by feline parvovirus (FPV; formerly FPL virus) and canine parvovirus (CPV), however, CPV infections in cats are uncommon (Barrs 2019). The FPL is also known to be the oldest known viral disease in cats wherein several epizootics that killed domestic cat populations in the 1800s could have been infected by FPV (Fairweather 1876, Barrs 2019) (Scott FW, 1987). Structure Fig. 01 A front view 60- meric assembly of FPV by Protein Data Bank in Europe containing 60 copies of Capsid protein VP1 ( https://www.ebi.ac.uk/pdbe/entry/pdb/1fpv ) The current taxonomic entity of FPV shares with CPV (Tattersall, 2006) wherein after crossing species barriers, CPVs evolved from FPV by acquiring five or six amino acid changes in the capsid protein gene (Truyen, 1999) (Appel, Scott et al. 1979, Black, Holscher et al. 1979, Osterhaus, van Steenis et al. 1980, Parrish 1990, Johnson and Spradbrow 2008, Stuetzer and Hartmann 2014, Barrs 2019). The causative agent FPV is a member of the genus Protoparvovirus in the family Parvoviridae with 5.2 kb long single stranded DNA genome, containing two open reading frames (ORFs): the first ORF encodes two non-structural proteins, NS1 and NS2; and the second ORF encodes two structural proteins, VP1 and VP2 (Reed, Jones et al. 1988, Zhou, Zhang et al. 2017). At first, FPV was thought not to infect cats (Truyen, Evermann et al. 1996). It replicates in thymus and bone marrow but not within the intestinal tract of dogs (Truyen and Parrish 1992, Truyen, Gruenberg et al. 1995). The pathway of viral entry into cells is not fully characterized, however through the feline transferrin receptor (TfR), FPV binds and uses the receptor to infect feline cells (Parker, Murphy et al. 2001, Hueffer, Govindasamy et al. 2003). However, CPV-2b and CPV-2c variants emerged, with only a single amino acid position different from CPV-2a, and infect cats both naturally and experimentally (Mochizuki, Horiuchi et al. 1996, Truyen, Evermann et al. 1996, Ikeda, Mochizuki et al. 2000, Nakamura, Sakamoto et al. 2001, Gamoh, Shimazaki et al. 2003, Decaro, Desario et al. 2011, Zhou, Zhang et al. 2017, Van Brussel, Carrai et al. 2019). FPV Infection The virus may be shed in feces even in the absence of clinical signs (subclinical infections), or before clinical signs are detected (Barrs 2019). The major portals of the FPV are the gastrointestinal (GI) tract and, less commonly, the respiratory tract. Generally, CPV is an uncommon cause of FPL and to date, no large-scale outbreaks of FPL have been confirmed to be caused by CPV (Barrs 2019). There were cases of indistinguishable CPV from FPV clinical signs in several cats (Mochizuki, Horiuchi et al. 1996, Miranda, Parrish et al. 2014, Byrne, Beatty et al. 2018, Barrs 2019). Moreover, coinfections of CPV and FPV were also reported in cats with clinical disease (Battilani, Balboni et al. 2011, Battilani, Balboni et al. 2013, Barrs 2019). The FPV can remain latent in peripheral blood mononuclear cells of healthy cats with high virus-neutralizing titers (Ikeda, Miyazawa et al. 1999, Miyazawa, Ikeda et al. 1999, Nakamura, Ikeda et al. 1999, Barrs 2019). The development of immunity of an unvaccinated cat to FPV is likely to increase with age (DiGangi, Levy et al. 2012). However, FPL mostly infects unvaccinated and incompletely vaccinated kittens. The age susceptibility correlates with the declining maternally derived antibodies (MDAs) as well as “the immunity gap” in incompletely vaccinated kittens (Barrs 2019). Clinical Signs/ Pathogenesis The FPV is resistant to heating (80C for 30 min) and low pH (3.0) (Goto, Yachida et al. 1974). Virions enter cells by endocytosis (Hueffer, Palermo et al. 2004). Viral DNA is released from the capsid and replicates through double-stranded RNA intermediates in the nucleus of the cell using the host’s DNA polymerase (Barrs 2019). It can be transmitted by the faecal-oral route and a contact with infected body fluids, faeces, or other fomites, as well as by fleas primarily spreads the virus. Viral replication primarily occurs in lymphoid tissue, bone marrow and intestinal mucosa in infected cats older than 6 weeks of age (Csiza, De Lahunta et al. 1971, Csiza, Scott et al. 1971, Parker, Murphy et al. 2001). Infection outcome ranges from subclinical to peracute infections with sudden death within 12 h (Stuetzer and Hartmann 2014). Initially, non-specific signs such as fever, depression, and anorexia during the acute stage (Addie, Jarrett et al. 1996). However, vomiting unrelated to eating occurs commonly and, less often, cats develop watery to haemorrhagic diarrhoea later in the course of disease, while some cats show extreme dehydration. Cats typically die of complications. Viral DNA can persist for long periods even after infectious virus has been lost, thus detection of DNA does not necessarily signify an active infection (Stuetzer and Hartmann 2014). Utero infection in early pregnancy can result in foetal death, resorption, abortion, and mummified fetuses while in later pregnancy may damage the neuronal tissue. The main clinical signs of FPV infection for new- born kittens include neurological, with ataxia, hypermetric movements and blindness, while some also shows signs of cerebellar dysfunction, forebrain damage (with seizures) with a range of severity and neurological signs. Although some kittens acquire MDAs, they can still get the virus for up to 2 months after birth (Csiza, Scott et al. 1971, Csiza, Scott et al. 1971, Stuetzer and Hartmann 2014). Infections occurring up to 9 days of age can also affect the cerebellum. Cats having mild cerebellar dysfunction may retain good quality of life. On the other hand, FPV can also cause retinal degeneration in infected kittens, with or without neurological signs (Percy, Scott et al. 1975, Stuetzer and Hartmann 2014). Diagnosis It is important to have the FPV detected early using accurate testing methods to prevent disease transmission
Canine HbA1c
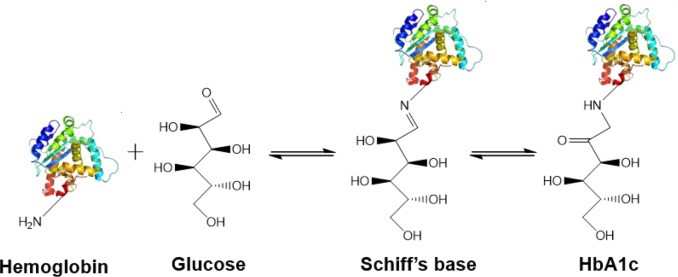
Canine HbA1c LIN, WEN-YANG (WESLEY), Ph.D HbA1c is a type of hemoglobin on which several monosaccharides such as glucose, galactose and fructose tend to bind with and exist in the bloodstream. The chemical linking process between sugar and hemoglobin is named glycation. HbA1c is actually an indicator of the beta-N-1-deoxy fructosyl on hemoglobin, which has been used as diagnostic measurement of long-term glycemic control for patients with diabetes mellitus. The increasing number of HbA1c in bloodstream represent the elevated plasma glucose level that usually indicating a poor diabetic management may lead to severer conditions. Due to the lifecycle of red blood cells is average four months, the HbA1c test could effectively present the real blood sugar degree in latest three months. In 1980, Wood and Smith first demonstrated the applicable method of examining canine diabetes with monitoring glycosylated haemoglobin. Later, Smith et al 1982, Mahaffey and Cornelius 1982, Dennis 1989, Jensen 1995 all confirmed this method is workable on diagnosing diabetic dogs. Molecular structure of HbA1c Figure 1 showed an aldimine linkage between glucose and hemoglobin (Figure1). Figure 1. Glycation process between Hemoglobin and glucose https://www.sciencedirect.com/science/article/pii/S0956566318304500 As we previously discussed that different monosaccharide would form different chemical structure with blood cells. Thus, while using cation exchange chromatography to separate Hemoglobin type A, different fractions would be separate out like HbA0, HbA1a, HbA1a2, HbA1b, and HbA1c. Fractions were named after eluting order (Figure 2). Figure 2. Different separated fractions of Hemoglobin type A with cation exchange chromatography https://www.sciencedirect.com/science/article/abs/pii/S0003269711000753 Glycated hemoglobin forming harmful factors in human body Highly reactive free radicals promote the formation of abnormal ferryl Hb (Fe4+-Hb), which enhance macrophage accumulation in blood vessel. Both macrophage accumulation and accumulation of glycated hemoglobin would elevate blood viscosity and slow down normal blood flow. Thus, atherosclerotic plaque would gradually occur in blood vessel. History of standardized HbA1c as diagnostic tool for diabetes Diabetes has become a serious health issue in entire world, due to over 220 million patients are suffering of it. Among all cases of diabetes, type 2 diabetes take majority part (90% to 95%). Besides, worsen type 2 diabetes could also cause extra complications, such as cardiovascular diseases, peripheral neuropathy, nephropathy, optic neuropathy, diabetic foot and even unto death. Several risks factor related to diabetes including high fat diet, obesity, smoking, elevated cholesterol levels, high blood pressure and lack of regular exercise. For preventing and relieving conditions of diabetes, taking healthy diet and regular exercise are important. In addition, diabetic patients’ blood sugar condition should be monitored regularly all the time. There are many clinical methods to evaluate glycemia like urine glucose, random or fasting plasma glucose etc. However, HbA1c is considered to be one of the most accurate and efficient method for measuring long-term blood sugar level (3 to 4 months). From 1894 to 1993, Diabetes Control and Complications Trial (DCCT) established the big data of diabetic patients’ HbA1c values to mean blood glucose resulted in making HbA1c a reliable index of mean blood glucose. However, DCCT haven’t standardized the HbA1c assay methods for labs and clinics. Later, the American Association for Clinical Chemistry (AACC) Standards Committee established a HbA1c Standardization Subcommittee to develop a plan for standardizing HbA1c assay that clinical laboratories could take advantage of it to perform precise glycemic evaluation and control. Now, the National Glycohemoglobin Standardization Program (NGSP) continue to develop reliable assays of HbA1c. Diagnosing canine diabetes with HbA1c 2018 American Animal Hospital Association suggested guidelines of diagnosis and assessment for animal diabetes. Clinical evaluation of animal diabetes (cats and dogs) include hyperglycemia, physical exam, complete blood count [CBC], Elevated blood glucose (BG), glucosuria, chemistry with electrolytes, urine analysis with culture, urine protein creatinine ratio (UPC), triglycerides, blood pressure (BP), and thyroxine (T4). While the level of BG concentration elevated to 200 mg/dL in dogs and 250–300 mg/dL in cats, glucosuria will typically occur. Pets with persistent glucosuria, persistent hyperglycemia, and presence clinical signs would be judged as diabetes mellitus (DM). Furthermore, HbA1c now have become a crucial glycemic indicator for diabetic dogs. Neslihan Tascene et al. revealed that blood HbA1c levels of diabetic were found to be 3.11±0.4 %, whereas the normal dog was 1.07±0.08 % respectively. Besides, the blood serum glucose level of diabetic dogs was around 526.71±22 mg/dl, whereas blood sugar in control dogs were around 97.80±2.93 mg/dl. Hasegawa S. demonstrated 6.41% HbA1c in diabetic dogs, whereas normal dogs with 2.6% (mean HbA1c of total Hb, %). And mean HbA1 values of normal dogs and diabetic dogs were 3.58 and 7.41%. In addition, Na-Yon Kim et al. showed significantly higher HbA1c concentrations of diabetic dogs (>6.2%) than non-diabetic dogs (p < 0.001) with commercial HbA1c testing system. Furthermore, Chao-Nan Lin et al. present the stability of canine glycosylated hemoglobin sample at room (25°C) and refrigerator (4°C) temperatures over 14 days. Besides, different purified methods would cause slight variation in measuring HbA1c value. Monitoring of pets’ diabetes Monitoring options include performance of blood glucose curves (BGCs), monitoring urine glucose (UG), measuring fructosamine, and assessment of clinical signs and weight. a. Blood glucose (BG) levels: Blood glucose levels fluctuate and could be used for indicating short periods of hyperglycemia. Normal BG were 63~110mg/dL in dogs; 47~151mg/dL in cats. As we mentioned, when the BG concentration goes over approximately 200 mg/dL in dogs and 250–300 mg/dL in cats, Glucosuria will occur. Blood Glucose Curves should be established during insulin treatment. b. Threshold of urine glucose (UG): UG concentration reflects only the average BG. Thus, it’s not recommended to solely rely on UG measurements is not recommended. Regardless, UG concentration can assist in assessment of DM together with other evaluation parameters. The threshold of urine glucose (UG) would fall in 180mg/dL in dogs; 252mg/dL in cats. c. Fructosamine: Fructosamine is a glycosylated protein formed by nonenzymatic, irreversible binding of glucose to serum albumin. It able to discern normal glycemic level from diabetes with chronic hyperglycemia and won’t be affected by
Case study: Feline infectious peritonitis in a cat presented because of papular skin lesions
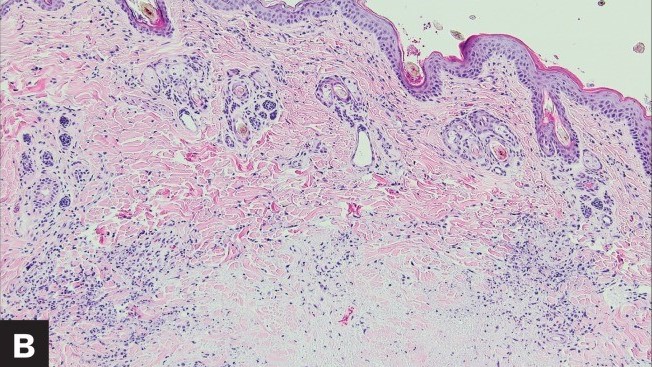
Case study: Feline infectious peritonitis in a cat presented because of papular skin lesions Robert Lo, Ph.D, D.V.M A 19-week-old neutered male domestic shorthair cat presented only multiple raised pruritic skin lesions along the dorsal head and back and no other symptoms. The cat showed poor appetite and spreading of the skin lesions five days after initial treatment, and then biopsies were taken and submitted to the dermatopathology service. Histopathology indicated strong suspicion of FIP. With cat’s health getting worse, euthanasia was performed. Then, necropsy was also performed. Abundant viscous serofibrinous effusions were found in the abdomen, thorax, and pericardium. Multiple white nodules were observed in the lungs, liver, and kidney. Histologic examination revealed multifocal to coalescing areas of pyogranulomatous inflammation in the affected tissues. Dermal necrosis was observed in skin sections. Immunohistochemical staining for intracellular feline coronavirus showed positive staining within the cytoplasm of macrophages in the lung, kidney, skin, and brain. Feline infectious peritonitis with associated cutaneous lesions was diagnosed in this cat based on gross and histologic lesions along with immunohistochemistry results. Figure 1: A — Skin lesions (multiple round raised skin nodules) on the dorsal surface of the head and neck. B —Dermal necrosis was observed in histological sections of skin; H&E. C —Immunohistochemical staining for intracellular feline coronavirus showed positive brown staining in skin section. (Redford T, Al-Dissi AN. Feline infectious peritonitis in a cat presented because of papular skin lesions., Can Vet J. 2019 Feb;60(2):183-185.) Original paper: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6340254/
Feline Immunodeficiency Virus (FIV): Virology, Diagnosis & Management
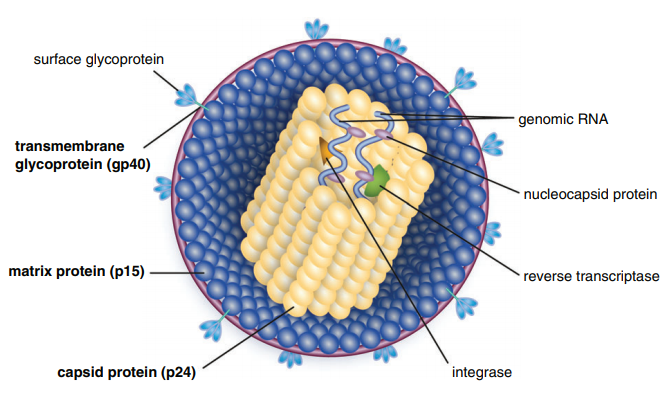
Table of Contents Maigan Espinili Maruquin 1. Introduction: The Scientific and Clinical Significance of FIV 1.1 Historical Discovery of FIV (1986) and Its Relevance as an HIV Model Feline Immunodeficiency Virus (FIV) was first recognized in 1986, when domestic cats in California presented with severe immunodeficiency syndromes remarkably similar to human AIDS. In 1987, Pedersen, Ho, and colleagues successfully isolated the virus, identifying it as a novel feline lentivirus and establishing the foundation for a new model of lentiviral immunopathogenesis (Pedersen, Ho et al. 1987). Initial observations emphasized that affected cats showed clinical signs of profound immune dysfunction—fever, lymphadenopathy, recurrent infections—but were negative for Feline Leukemia Virus (FeLV), differentiating this syndrome from previously known retroviral diseases in cats (Harbour, D.A. et al. 2004). Because FIV belongs to the Lentivirus genus within Retroviridae, similar to Human Immunodeficiency Virus (HIV), it was subsequently named in parallel with HIV. The biological, genomic, and pathological similarities between FIV and HIV—including their shared tropism for CD4⁺ T lymphocytes, slow progression, and lifelong persistence—positioned FIV early on as an important natural animal model for studying human AIDS (Elder & Phillips 1995; Miller, Cairns et al. 2000; Troyer, Pecon-Slattery et al. 2005; Hosie, Techakriengkrai et al. 2017). To this day, FIV remains a critical comparative model for HIV vaccine development, antiviral therapy research, and studies of lentiviral immune dysfunction. 1.2 Classification as a Lentivirus and Early Pathogenesis Studies FIV is a member of the Lentivirus genus, characterized by chronic, progressive infections and slow viral replication cycles. Like HIV, FIV integrates its genome into the host’s DNA to form a provirus, resulting in lifelong infection (Bendinelli, Pistello et al. 1995; Westman, Malik et al. 2019). Three Major Clinical Stages of Infection FIV infection progresses through a series of well-defined clinical stages that mirror HIV pathogenesis: Acute (Primary) Infection Occurs 1–6 weeks post-exposure and may last 1–4 weeks. Common findings include:• transient fever, lethargy, dullness• generalized lymphadenopathy• transient neutropenia• anorexia, diarrhea• mild upper respiratory signs(Ishida & Tomoda 1990; Bendinelli, Pistello et al. 1995) During this phase, the thymus and lymphoid tissues are important early replication sites where lesions may appear within 4 weeks (Harbour, D.A. et al. 2004). Asymptomatic (Latent) Phase Often lasts 1.5–2 years or longer.• Cats appear healthy but remain persistently infected.• Viral replication continues at low levels.• Subclinical chronic inflammatory lesions may occur (Harbour, D.A. et al. 2004). Terminal Immunodeficiency Phase (Feline AIDS; FAIDS) Characterized by:• severe immune collapse• high viral replication• chronic secondary infections• neoplasia (especially lymphomas)• neurological abnormalities(Pedersen 1993; Bendinelli, Pistello et al. 1995; Westman, Malik et al. 2019) These stages reflect lentiviral dynamics nearly identical to HIV progression in humans. 1.3 Distinction Between FIV and FeLV in Naturally Infected Cats Although both FIV and FeLV are important feline retroviruses, they differ significantly in classification, transmission, and pathogenesis: Classification and Molecular Differences FIV: Lentivirus; elongated morphology; Mg²⁺-dependent reverse transcriptase (Harbour, D.A. et al. 2004). FeLV: Gammaretrovirus; Mn²⁺-dependent reverse transcriptase. Transmission FIV: Primarily transmitted via bite wounds during fighting or mating aggression (Perharic, Bidin et al. 2016; Miller, Boegler et al. 2017). FeLV: Transmitted via casual contact: grooming, shared bowls, and vertical passage. Clinical Outcome FIV:• Lifelong persistent infection• Slow immunodeficiency progression• Many cats live normal or near-normal lifespans with appropriate care FeLV:• More acutely pathogenic• Causes immunosuppression, anemia, lymphoma• Can manifest as abortive, regressive, or progressive infection These differences require distinct diagnostic and management approaches. 1.4 Why FIV Remains a Critical Feline Pathogen in Modern Veterinary Medicine Global Prevalence and Risk Factors FIV prevalence varies with geography, lifestyle, and demographics:• 2.5–5 percent among healthy cats in North America• Up to ≥15 percent in high-risk populations (Bendinelli, Pistello et al. 1995)• Higher in older, free-roaming, intact male cats due to aggressive behaviors Pathogenesis: Progressive Immune Dysfunction FIV induces a chronic decline in immune function via:• depletion of CD4⁺ T cells• inversion of the CD4:CD8 ratio• chronic immune activation• susceptibility to secondary infections These immune abnormalities closely mirror HIV-induce immunopathology. Neoplastic and Neurological Sequelae FIV markedly increases the risk of neoplasia, especially B-cell lymphosarcoma.Neurological manifestations include:• seizures• altered behavior• dementia• ataxia(Ishida & Tomoda 1990; Bendinelli, Pistello et al. 1995) Transmission Challenges Although FIV is shed in saliva, deep bite wounds remain the dominant transmission route. Viral RNA, DNA, and antibodies have been detected in saliva and oral tissues (Yamamoto, Sparger et al. 1988; Pedersen, Yamamoto et al. 1989; Miller, Boegler et al. 2017). Ethical and Clinical Implications Because many FIV-positive cats remain healthy for years:• A positive test result must never be the sole basis for euthanasia (Harbour, D.A. et al. 2004).• Appropriate counseling is required to prevent mismanagement.• Early diagnosis enables interventions that improve longevity and quality of life. Importance in HIV Research The strong biological parallels between FIV and HIV continue to position FIV as a unique model for:• immune dysfunction• neurological disease• vaccine development• antiretroviral therapy testing(Hosie, Techakriengkrai et al. 2017) 2. Virology and Molecular Biology 2.1 Viral Structure Feline Immunodeficiency Virus (FIV) is a member of the Lentivirus genus within the Retroviridae family. Mature virions measure approximately 100–110 nm in diameter and contain a characteristic cylindrical nucleocapsid core. The virion is enclosed by a host-derived lipid bilayer acquired during budding from the plasma membrane of infected cells. Embedded within this lipid envelope are short surface glycoprotein spikes that are fundamental to viral infectivity and immune recognition. The viral Env glycoprotein precursor undergoes proteolytic cleavage to produce two mature subunits: Surface unit (SU, gp95) Transmembrane unit (TM, gp40) These subunits are non-covalently associated in a trimeric structure within the viral membrane. Together, they mediate: Receptor recognition and binding Membrane fusion and viral entry Immune evasion and antigenicity, serving as major targets for neutralizing antibodies The gp40 transmembrane glycoprotein is a key antigen detected by many point-of-care diagnostic kits, underscoring its immunological relevance. Within the core, the virion contains two identical positive-sense RNA strands assembled with nucleocapsid proteins, essential viral enzymes, and structural components. This genome–nucleoprotein complex is enclosed by the capsid protein (CA, p24), itself surrounded by the matrix protein (MA, p14)
Canine babesiosis
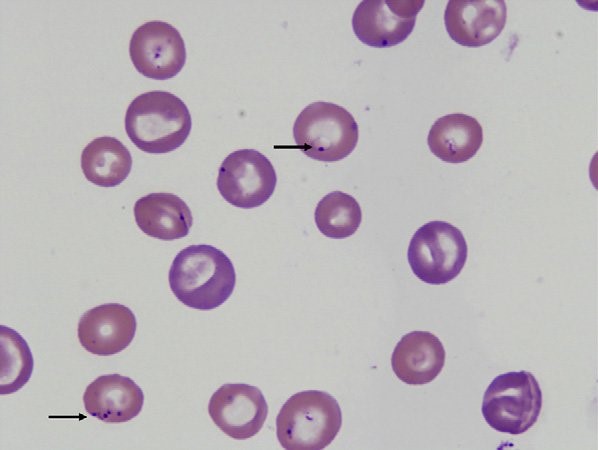
Canine babesiosis Robert Lo, Ph.D, D.V.M Canine babesiosis occurs worldwide and results from infections with a variety of Babesia spp., tick-borne hemoprotozoa. The disease was first described in cattle with hemolytic anemia in 1888 by a Rommanian bacteriologist, Victor Babes (Babes, 1888). Babesiosis is one of the most important tick-borne infectious diseases of domestic and wild mammals and still poses significant diagnostic and therapeutic challenges for veterinary practitioners around the world. More than 100 Babesia spp. were reported in vertebrate hosts (El-Bahnasawy et al., 2002). With the expansion of tick habitats, the spread of parasites into new geographical areas has been an increasing problem worldwide. The Babesia genus belongs to the order Piroplasmida in the phylum Apicomplexa and can be seen as non-pigment forming pear or signet-ring shaped organisms in mammalian erythrocytes. Asexual reproduction occurs in canine erythrocytes while the sexual conjugation and the sporogony stages of their life-cycles occurs in a variety of hard ticks, which can transmit the organism transovarially. Based on their morphology, Babesia are classified into the small Babesia group (trophozoites of 1.0-2.5 µm; including B. gibsoni, B. microti, and B. rodhaini), and the large Babesia group (2.5-5.0 µm; including B. bovis, B. caballi, and B. canis). Etiology and epidemiology Dogs are mainly infected by two species of Babesia: B. canis and B. gibsoni though they can also be infected by several other species of Babesia. Babesia canis has a piriform (teardrop) shape and frequently more than one merozoite is found in a single erythrocyte (Fig. 1). Babesia gibsoni is more pleomorphic (usually oval or signet-ring shapes) (Fig. 2). Fig. 1. Two pear-shaped Babesia canis organisms in an erythrocyte. (Duh et al., 2004) Fig. 2. Babesia gibsoni in erythrocytes in a blood smear stained with modified Wright technique. (Trotta et al., 2009) Babesia canis was further categorized into three subspecies (B. canis canis, B. canis rossi, B. canis vogeli) on the basis of cross-immunity, serological testing, vector specificity and molecular phylogeny (Uilenberg et al., 1989). These three subspecies of babesia are significantly different in their clinical presentation, geographical distribution and vector specificity. With the advent of molecular phylogenetic analysis, in particular that of the 18S rRNA gene, these subspecies are now considered to be separate species (Carret et al., 1999; Lack et al., 2012; Zahler et al., 1998). Recently an unnamed fourth “large” Babesia sp. (coco) has been found in dogs in North Carolina in the US (Birkenheuer et al., 2004) and has caused babesiosis in immunocompromised dogs (Sikorski et al., 2010). The small Babesia are more genetically closer to Theileria spp. than to Babesia spp. based on study of the 185 rRNA gene locus. Babesia canis, transmitted by Dermacentor reicultatus, is the most common pathogen of canine babesiosis in temperate regions of Europe and has been reported sporadically around the world (Solano-Gallego wt al., 2011). Most of clinical cases are reported in spring and autumn, which is associated with the seasonal activity of tick vector (Solano-Gallego et al., 2011; Matijatko et al., 2012). Babesia vogeli, transmitted by Rhipicephalus sanguincus, is a less pathogenic species and is found not only in tropical and subtropical regions but also in colder areas (Cassini et al., 2009). Babesia rossi, transmitted by Haemaphysalis elliptica (syn. Haemaphysalis leachi) (Penzhorn, 2011), is the most virulent species among large Barbesia species and is endemic in southern Africa but has been reported in other regions of eastern and southern Africa (Oyamada et al., 2005). Babesia gibsoni, a virulent parasite in dogs of all ages, is endemic in Asia and occurs sporadically in the rest of the world. Ticks of the complex R. sanguineus may serve as potential vectors for B. gibsoni, at least in Europe, while in Asia, its main distribution range is attributed to transmission by the tick Haemaphysalis longicornis (Hatta et al., 2013; Iwakami et al., 2014). In addition, B. gibsoni can be transmitted by blood exchange when dogs fight (Irwin, 2009). Transmission Babesia spp. are mainly transmitted through tick bites and can infect a wide variety of domestic and wild animals as well as humans (Schnittger et al., 2012). Hard ticks are the main vectors for Babesia spp.; within the tick, Babesia spp. undergo the sexual stage in the tick gut is followed by sporogony in its tissues. The parasite then reaches the tick salivary glands. A blood meal will ultimately transmit the sporozoites from the tick’s salivary gland to their new vertebrate host (Chauvin et al., 2009), where the protozoan undergoes asexual replication (merogony) within the red blood cells. Babesia spp. are transmitted both transstadially and transovarially (Chauvin et al., 2009). Pathogenesis and clinical signs After sporozoites enter the red blood cells, Babesia multiply via repeated binary fission, resulting in up to 16 merozoites. The parasites induce FLP (fibrinogen like proteases) that cause the red blood cells to become sticky, resulting in capillary sludging. Parasitized cells are sequestered in the spleen, and extravascular and intavascular hemolysis occurs. The incubation period following tick transmission is 10-21 days. The clinical picture is similar for all Babesia infections, whether they involve large or small Babesia. Pathogenicity is more severe in young dogs, immunosuppressed dogs, heavily parasitized dogs, and when there is exposure to a virulent strain or concurrent infection (Hunfeld et al., 2008; Matijatko et al., 2012; Schetters et al., 1997; Solano-Gallego L et al., 2008). Infected dogs may exhibit either peracute, acute, or subclinical signs of disease (Freeman et al., 1994; Jacobson, 2006; Wlosniewski et al., 1997). Peracute signs include acute onset of hypotensive shock, vasculitis, extensive tissue damage, hypoxia, and death. Signs of acute disease include fever, lethargy, hemolytic anemia, thrombocytopenia, splenomegaly, lymphadenopathy, icterus, and hemoglobinuria. Less common signs include ascites, peripheral edema, ulcerations, stomatitis, gastroenteritis, CNS signs, acute renal failure, and rhabdomyolysis. Acute infections of virulent strains of B. canis have been associated with induction of the systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) secondary to massive immune
Feline corona virus (FCoV) and Feline infectious peritonitis (FIP)
Feline corona virus (FCoV) and Feline infectious peritonitis (FIP) Andy Pachikerl, Ph.D Introduction: Feline coronavirus (FCoV) is a widely known positive-stranded RNA virus is infecting many cats worldwide (Satoshi, Takehisa and Motonobu 2012). This virus belongs to the species Alphacoronavirus 1 of the genus Alphacoronavirus from within the virus family Coronaviridae (Antoniw and Adams 2013). Alphacoronavirus 1 also includes the canine coronavirus (CCoV), which has been previously reported (link) and the porcine transmissible gastroenteritis coronavirus (TGEV) (Satoshi, Takehisa and Motonobu 2012). There are just two different forms of FCoV namely, the FECV (feline enteric coronavirus) that infects the intestines and FIPV (feline infectious peritonitis virus) that causes the disease feline infectious peritonitis (FIP). Feline coronavirus can typically be found in feces of infected cats and it can be transmitted to healthy cats via coming in contact by means of the fecal-oral route (Hartmann, Feline infectious peritonitis 2005). In environments with multiple cats, the transmission rate is higher as compared to a single-cat environment (Satoshi, Takehisa and Motonobu 2012). The virus is actually symptomless and insignificant until specific mutations occur that can cause complications such as FIP (Satoshi, Takehisa and Motonobu 2012). FIPV is the complication that can be the result of FCoV and causes FIP in cats, for which treatment is generally symptomatic and palliative only. The drug GS-441524 shows promise as an antiviral treatment for FIP, but now it’s still under further research development. Virology Feline enteric coronavirus (FECV) This is caused when the coronavirus becomes prominent in the mature gastrointestinal epithelial cells i.e. enterocytes, brush border, microvilli and villi of the cat (Rottier Peter, et al. 2005). This intestinal infection can show some outward symptoms and is usually chronic. The virus is excreted in the faeces of the symptomless carrier and can solely be detected by polymerase chain reaction (PCR) of faeces or by PCR testing of rectal samples. Cats that are raised in group can infect one another or each other with various strains of the virus. Some cats do show resistance to the virus and are not infected or even become a carrier, while others may become a FECV carrier (Rottier Peter, et al. 2005). Carriers may heal spontaneously but acquired immunity may be short and they may go on to being reinfection. Usually within a few weeks, if they are living in a group with healthy but persistent, excretory carriers; some cats will never heal, and the excretory phase remain permanent. Feline infectious peritonitis (FIPV) and Feline infectious peritonitis (FIP) The virus can further complicate into what is known as FIPV when random errors occur during when the virus infects an enterocyte, causing the virus to mutate from FECV to FIPV (Rottier Peter, et al. 2005). FIPV causes lethal, incurable disease such as: feline infectious peritonitis (FIP). Prior to being domesticated, cats are solitary animals and do not like to share space (i.e. hunting areas, rest areas and defecation sites). Domestic cats living in group therefore have a much higher epidemiological risk of mutation. After this mutation, the FCoV acquires a tropism for macrophages while losing intestinal tropism (Rottier Peter, et al. 2005). Regardless of the source of FIPV and uncertainty about the significance of genetic differences, the relationship between virulence and macrophage/monocyte tropism has been firmly established (Pedersen 2009). While both FIPV and FECV may cause viremia (Gunn-Moore, Bruffydd-Jones and Harbour 1998, Febr, et al. 1996), only FIPV replicates in macrophages and causes the disease (Vennema, et al. 1998, Stoddart and Scott 1989). Complex immune reactions between the virus, antiviral antibodies, and complement cause disseminated vasculitis, which is the hallmark of FIP (Hartmann 2005, Pedersen 2009). In a large group of cats, n, the epidemiological risk of mutation is higher and expressed theoretically as: E = n2 – n. A house hosting 2 cats therefore has a risk of E = 2. When 4 kittens (6 in total) are born into this group, the risk increases from 2 to 30. Overcrowding increases the risk of mutation and conversion from FECV to FIPV, which constitutes a very high-risk factor for the development of FIP cases. FIP has been shown to develop in cats whose immunity is low, such as younger kittens, old cats, immunosuppressed due to viral-FIV (feline immunodeficiency virus) and/or FeLV (Feline leukaemia virus) and stress such as: separation and adoption (Rottier Peter, et al. 2005). The incidence of disease is bimodal, occurring most commonly in cats younger than 18 months and older than 12 years of age. There is a genetic component that contributes to the risk of developing FIP, thus littermates of kittens that have developed FIP are at increased risk. Unfortunately, there is no way to predict, out of a group of FCoV seropositive cats at risk for FIP, which ones are most likely to develop the disease. Infection of macrophages by FIPV is responsible for development of a fatal granulomatous vasculitis, or FIP (see granuloma). Development of FIP depends on two factors: virus mutation and low immunity where virus mutation depends on the rate of mutation of FECV to FIPV and the immune status depends on the age, gene pool and the stress level of the cat. High immune status will be more effective at slowing down the virus (Rottier Peter, et al. 2005). How does FCoV cause FIP? Infections with FCoV are usually limited to the intestinal tract, with very limited viral replication elsewhere. Strains of FCoV causing these infections are referred to as feline enteric coronavirus (or FECV). During infection, and while the virus replicates in the intestine, it undergoes spontaneous mutations. This leads to the development of different strains of the virus, and occasionally a strain may develop that has dramatically altered disease-causing potential – this viral strain is referred to as feline infectious peritonitis virus (FIPV). FIPV strains of FCoV differ from FECV in that they no longer replicate well in the intestine, but rather preferentially infect macrophages – one of the important cells of
Giardia lamblia
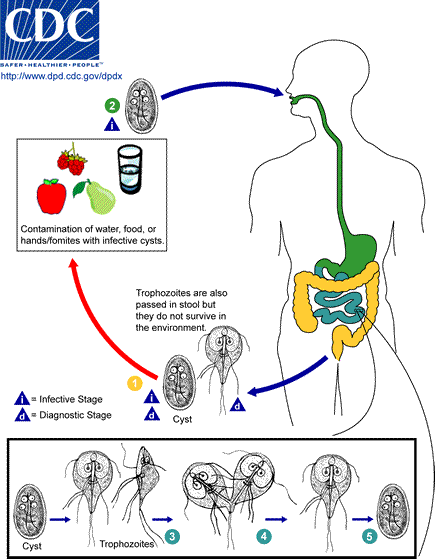
Giardia lamblia LIN, WEN-YANG (WESLEY), Ph.D Giardia lamblia (=G. intestinalis, =G. duodenalis) also called Giardia duodenalis, Giardia intestinalis and pear-shaped flagellate is a common and well-known anaerobic flagellated protozoan parasites colonize in human (or in canine) small intestines and cause diarrhea, stomach pain etc. Its character of parasitic zoonoses make them also infecting other mammalian such as mice, rabbits, birds, reptiles and amphibians. Giardia lamblia prefer to live in cold water like rivers in mountains, cold springs and contaminated pools, which became the major cause of diarrhea in the US. Traveling in the developing world, dinning food without proper cooking, changing diapers etc. would be major risk factors of Giardiasis. The stool tests is the major diagnosis tool. Discovery In 1681, the Dutch scientist Antonie van Leeuwenhoek first found giardia through microscope. In 1915, Giardia lamblia was officially named after scientist Alfred Mathieu Giard who further studied it. In 2010, Andersson et al. has sequenced Giardia’s genome and discovered its ~5000 genes with 11.7 million base pair building blocks. Cellular and physiological properties Giardia is a diplomonad with two nuclei and duplicate organelles follow by four associated flagella and without cytostomes, Golgi apparatus and mitochondria. However, they have a mitochondrial remnant, mitosomes, which take part in the maturation of iron-sulfur proteins rather than ATP synthesis. The life cycle of Giardia lamblia consist of reproductive phase and resting phase that present two different forms: a swimming trophozoite and an infective cyst (Figure 1). Genotyping of Giardia lamblia sub-classified eight genetic assemblages (from A to H). Among eight assemblages, A and B infect are the most dominate assemblages that infect wide range of species including human being. Various species of Giardia exist in different kind of hosts were identified by PCR or genetic tools include G. ardeae and G. psittaci from birds, G. agilis from amphibians, G. microti from voles and G. muris from other vertebrates. Epidemiology In 2013, WHO estimated that there were about 280 million people diagnosed with the Giardia infection around the world. The prevalence rate of Giardia in developed countries was 5% and over 20% among developing countries. In 2018, it present that 15,584 reported cases in 3–7% prevalence of the population in the US, especially high incidence in California, New York, Florida, and Wisconsin. Besides, the highest incidence months of giardiasis would be July, August, and September in the America. Furthermore, 23 of the 31 countries in the Europe had reported total 17,278 confirmed giardiasis cases in 2014. Thus, Giardiasis become one of the top 10 parasite diseases in human beings. Risk factors Travelers to countries where giardiasis is common. Contacting contaminated drinking water, lakes or rivers, animals who have the disease. Giardiasis often happened in the summer because of the higher activities rate in the wilderness. Pathophysiology Giardia exist in rivers, streams, wells and pools. It infect humans or animals by their contacting of contaminated foods, water, contaminated feces and sniffing unclean ground. It is reported that people (or pets) infected with Giardia may have no symptoms, but still spread the disease. Animals such as canines, cows, rodents, beavers, and sheep are also infecting targets of Giardia lamblia. Figure1. The life cycle of Giardia lamblia ( http://www.dpd.cdc.gov/dpdx/images/ParasiteImages/G-L/Giardiasis/Giardia_LifeCycle.gif ) Giardia could transform between cyst form and trophozoite form. It spread and infect host by the cyst form that can remain contagious in cold water up to 3 months. After arriving intestines, Giardia stick to intestinal wall and colonize in the gut by altering its appearance into trophoziote form. Furthermore, they would recover back to cyst form when excreting out with stools. Giardia is spreading through fecal-oral route by animals’ contacting of contaminated water and food. It is one of the most common waterborne outbreaks of diarrhea in the America. Giardia spread between people and animals (Figure 1). When sojourning in the gastrointestinal tract with the trophoziote form, Giardia would engage in inhibiting of brush border enzymes that involved assimilation of disaccharide sugars, altering microvillus’ morphology that cause poor absorption of nutrients and water, triggering apoptosis of intestinal epithelial cells and enhancing intestinal permeability. Eventually, they would cause several clinical symptoms include diarrhea and intestinal malabsorption with or without histological changes. For increasing intestinal permeability, Giardia lamblia would take several dedicated process such as assisting proliferation of crypt cells and using enzymes to degrade proteins on the villi of the brush border. The degraded proteins would likely lead to immunological recruitment and activation of host T lymphocytes on endothelial cells for removing injured cells. In addition, Giardia triggered cell apoptosis by downregulating of the anti-apoptotic Bcl-2 and upregulating of the proapoptotic Bax would facilitate breaking down intestinal barrier and enhancing permeability. They could also execute tactics of consuming all local arginine for decreasing the formation of the gas nitric oxide and protecting its own development. Signs and symptoms The parasitic disease caused from the infection of Giardia, called Giardiasis, also known as beaver fever. A 10% of Giardiasis can be temperate and showed no sign, which recovery by host’s immune function without extra treatments. Nevertheless, 90% infected hosts show apparent symptoms 2 days after infection and last 1 to 6 weeks. Giardia would make atrophy in the small intestinal villi that would lead to foul- smelling diarrhea and cause dehydration accompanied with malnutrition. Diarrhea is the most common and dominant sign (in both humans and animals) accompanied by other symptoms include excess gas, abdominal cramps, abdominal pain, weight loss, nausea, vomiting, bloody feces and fever. However, only about 15% of infected hosts would occur fever. Most canines would be less active instead of exhibiting fever. Lesser common cases shows signs beyond intestinal system as itchy skin, hives and swelling of the eyes and joints. Medication should be administrated as soon as possible. Chronic diarrhea could last for weeks or months if untreated. Symptoms such as post-infectious irritable bowel syndrome, lactose intolerance and food allergies may occur and remain even if Giardia were dealt with medication. Diagnosis Detecting antigens in stool specimens, which
