Bird Sexing

Bioguard Corporation Many mammals are sexually dimorphic, which means that their sex can be identified based on their appearance. However, it is not the case for most birds. Many birds are sexually monomorphic although males and females may differ markedly in size, color, and appearance in some birds, such as peacocks (Fig. 1). Knowledge of a bird’s gender is important for veterinary practitioners, ornithologists and aviculturists. Gender identification in birds is beneficial for proper pairing of birds, and preparing owners for the eventual changes their bird will experience as it matures. Furthermore, knowing the gender of a bird allows veterinarians to diagnose gender-specific diseases. Traditional sexing The traditional methods used to identify a bird’s gender include behavioral observation, morphological differences, acoustic analysis, laparoscopic examination, laparotomy, vent sexing (cloacal examination), steroid sexing, and chromosome inspection (karyotyping). These methods can be inaccurate, invasive, or costly. As an alternative to these methods, molecular DNA-based sexing methods provide fast, accurate and non-harmful to the bird. DNA sexing The XY sex-determination system is used to classify many mammals, some insects (Drosophila), and some plants (Ginkgo tree). In this system, most females have two of the same kind of sex chromosome (XX), while most males have two distinct sex chromosomes (XY). The sex of birds is determined by the ZW sex determination system. Females have the heterogametic sex chromosomes ZW, whereas males have the homogametic ZZ. The standard method of molecular sexing in birds relies on PCR amplification of non-coding sequences (introns) located in the sex-chromosome-specific gene encoding the Chromo-Helicase-DNA binding protein (CHD1), and mapping on the sexual W (CHD-W) and Z chromosomes (CHD-Z). The length polymorphisms between CHD-W and CHD-Z alleles allows for sex discrimination. Amplification of the CHD gene leads to a single amplicon in males and two distinct amplicons in females. In addition to PCR, other PCR-based technologies have been applied to avian sexing, including Single Strand Conformation Polymorphism (SSCP), Restriction Fragment Length Polymorphism (RFLP), and Amplified Fragment Length Polymorphism (AFLP). These methods, however, are not viable for commercial use. Conclusion DNA sexing provides over 99.99% accuracy in determining a bird’s gender. This testing allows bird owners to quickly and accurately identify their birds’ gender, thereby enhancing the efficiency of pairing for breeding and conservation efforts.
Polyomavirus in Birds
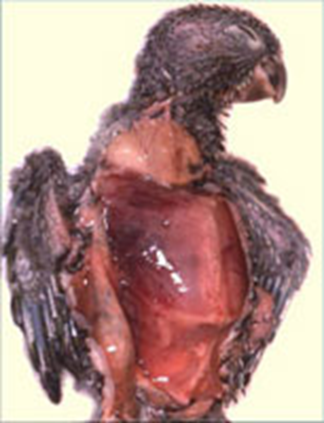
Long Pham Avian polyomavirus in birds poses a significant concern for caged birds, leading to substantial economic losses for pet retailers and bird enthusiasts. Initially identified in budgerigars during the early 1980s in Ontario, Canada and in the southern regions of the United States, it was initially termed Budgerigar Fledgling Disease Virus. This nonenveloped DNA virus belongs to a single genus Polyomavirus in the family Polyomaviridae. Further research revealed its ability to infect various species of psittacine birds, leading to its designation as avian polyomavirus. Avian polyomavirus is widespread, affecting psittacine birds in numerous countries, making it a notable concern in aviculture. Transmission Polyomavirus is typically transmitted through direct contact with infected birds. Additionally, transmission can occur through contact with contaminated feces, dander, air, nest boxes, incubators, feather dust, or from infected parent birds passing it to their chicks. Clinical signs Young birds, ranging from newborns to juveniles (14-56 days), are particularly vulnerable to polyomavirus infection, which is often fatal. Following infection, it typically takes 10-14 days for birds to display symptoms. However, some birds may remain asymptomatic. If symptoms do appear, the bird’s death may be imminent, often occurring within one or two days. The virus compromises the bird’s immune system, making it susceptible to other pathogens such as viruses, bacteria, fungi, and parasites, leading to secondary infections and eventual demise. Common symptoms of polyomavirus infection in birds include lethargy, regurgitation, cutaneous hemorrhage, abdominal distention, excessive urination, diarrhea, tremors, and feather abnormalities. Diagnosis The typical antemortem diagnosis involves using PCR on cloacal swabs, blood samples, and affected feather samples. It also includes conducting virus-neutralizing antibody tests on blood samples to identify birds that have previously been exposed to the virus. In a flock setting, diagnosis is commonly based on clinical observations, the characteristics of the affected birds, and findings from necropsies. Treatment and Prevention Aviary management strategies include avoiding housing budgerigars or lovebirds with other species, maintaining strict hygiene protocols, restricting access to the nursery, and enforcing a 90-day quarantine with testing before introducing new birds. Removing Avian Polyomavirus (APV) from an infected budgerigar aviary is complex and involves stopping breeding for six months. During this period, infected neonates, fledglings, and adult birds must be isolated while the aviary undergoes thorough disinfection. Nest boxes should be disinfected or replaced. After six months, breeding can resume in a sanitized environment. Preventive measures for pet stores include separating neonates from different sources, sourcing birds from facilities conducting polyomavirus testing and vaccination, and ideally avoiding transactions involving unweaned birds. References Bernier, G., M. Morin, and G. Marsolais. 1981. A generalized inclusion body disease in the Budgerigar (melopsittacus undulatus) caused by a papovavirus-like agent. Avian Diseases 25:1083-1092. Latimer, K. S., F. D. Niagro, R. P. Campagnoli, B. W. Ritchie, D. A. Pesti, and W. L. Steffens. 1993. Diagnosis of concurrent avian polyomavirus and psittacine beak and feather disease virus infections using DNA probes. Journal of the Association of Avian Veterinarians 7:141–146. Samanta, I., and S. Bandyopadhyay. 2017. Infectious diseases. Pet bird diseases and care 13–166.
Severe Fever with Thrombocytopenia Syndrome
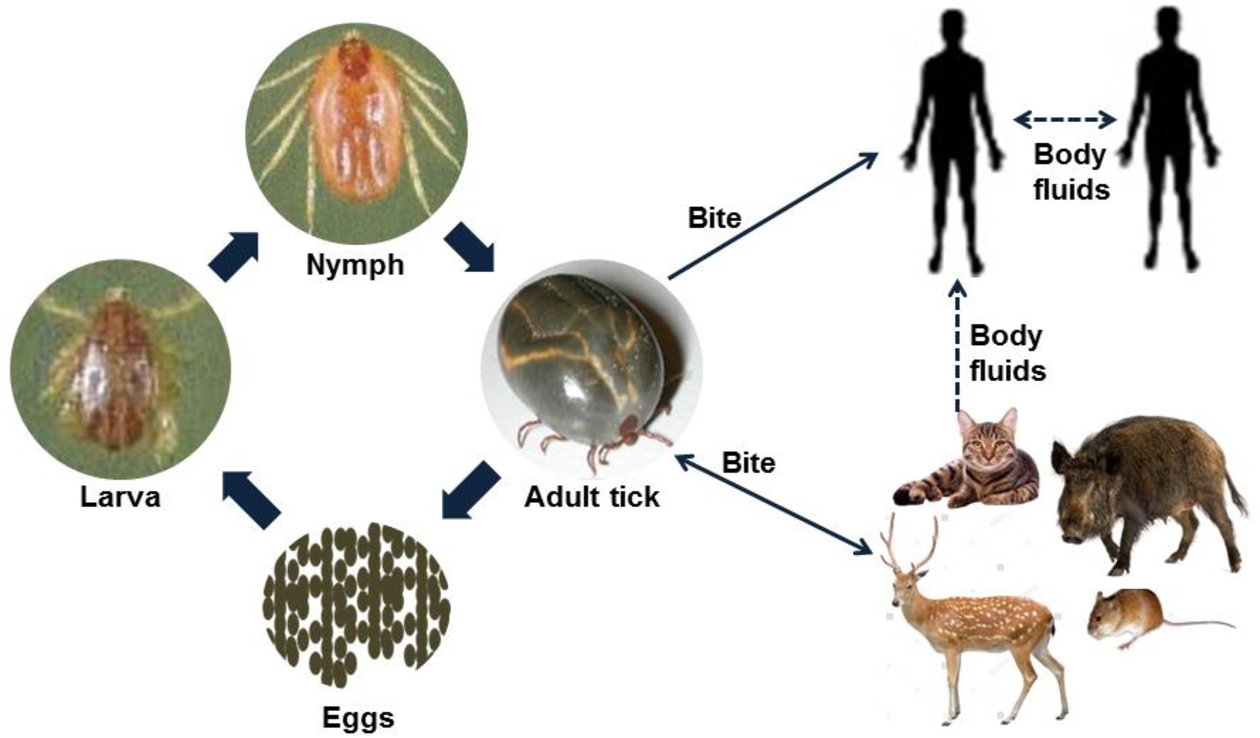
Bioguard Corporation Severe Fever with Thrombocytopenia Syndrome (SFTS) is an emerging infectious disease that was first reported in China in 2011. It is caused by the Severe Fever with Thrombocytopenia Syndrome virus (SFTSV) and is transmitted to humans and animals through the bite of an infected tick. The main clinical symptoms of SFTS include fever, vomiting, diarrhea, multiple organ failure, thrombocytopenia, leucopenia, and elevated liver enzyme levels. SFTSV has been detected in both domestic and wild animals, although most vertebrate animals were found to be sub-clinically infected with SFTSV. Pathogen SFTSV is a tick-borne virus belonging to the Genus Bandavirus (former Huaiyangshan Banyangvirus), Family Phenuiviridae. Vector and Disease Transmission The exact lifecycle and transmission mechanisms of SFTSV are not fully understood. However, ticks, particularly the Asian longhorned tick (Haemaphysalis longicornis), are believed to be the primary route of transmission. Other ticks, including Rhipicephalus sanguineus and Haemaphysalis concinna, have also been found to carry SFTSV. This suggests that ticks play a significant role in the transmission of SFTSV, similar to other members of the Phenuiviridae family, which are also known to be vector-borne. SFTSV Transmission Ticks are believed to be the primary vectors for SFTSV transmission, mainly through their bites. However, the virus can also be transmitted by infected animals or humans. Transmission of SFTSV from infected companion animals, such as cats and dogs, to humans has been reported through various routes. Pathogenesis Cytokine storm is thought to play a crucial role in the pathophysiology of SFTS. During the acute phase of the disease, the release of cytokines triggers a systemic inflammatory response. Histopathological examination of lymph nodes reveals necrosis and hemophagocytosis. The virus can be detected in the liver, spleen, adrenal glands, bone marrow, and lymph nodes. Thrombocytopenia may occur due to viral attachment to platelets, which are subsequently cleared by the spleen. SFTSV and Animal SFTSV has been detected in both domestic and wild animals; however, most vertebrate animals appear to be sub-clinically infected. Previous research indicates that antibodies (IgG/IgM) against SFTSV were found in goats and sheep (45.70%), cattle (36.70%), dogs (27.00%), chickens (9.60%), pigs (3.20%), and rodents (3.20%). A survey conducted in Taiwan identified SFTSV in Rhipicephalus microplus ticks collected from cows and goats, and antibodies against SFTSV were also detected in goats. In experimental studies, four out of six cats infected with SFTSV died, displaying symptoms as severe or more severe than those observed in human patients (Scientific Reports, 2019). Additionally, two captive cheetahs in a Japanese zoo died of SFTS in 2017, and there have been reports of fatal SFTSV cases in cats in Japan. References Casel MA, Park SJ, Choi YK. Severe fever with thrombocytopenia syndrome virus: emerging novel phlebovirus and their control strategy. Exp Mol Med. 2021 May;53(5):713-722. Seo JW, Kim D, Yun N, et al. Clinical Update of Severe Fever with Thrombocytopenia Syndrome. Viruses. 2021 Jun 23;13(7):1213. Sharma D, Kamthania M. A new emerging pandemic of severe fever with thrombocytopenia syndrome (SFTS). Virusdisease. 2021 Jun;32(2):220-227.
Cryptosporidiosis

Bioguard Corporation Cryptosporidiosis is an illness you get from the parasite Cryptosporidium. It causes watery diarrhea and other gastrointestinal (gut) symptoms. In addition to stomach infection, this parasite can infect the respiratory system causing a cough and/or problems breathing. The family Cryptosporididae belongs to the phylum Apicomplexa characterized by an anterior (or apical) polar complex (with apical rings, micronemes, and subpellicular microtubules), which allows penetration into host cells. Cryptosporidium species are able to infect a broad range of hosts including humans, domestic and wild animals (mammals, birds, fish, marsupials, reptiles, and amphibians) worldwide. Transmission and Life Cycle Humans and animals become infected with Cryptosporidium by touching anything that has come in contact with contaminated feces, although the most common mode of transmission is represented by ingestion of oocysts in contaminated food and water or air. Cryptosporidium has three developmental stages: meronts, gamonts, and oocysts. They reproduce within the intestinal epithelial cells. Two types of oocysts, thick-walled and thin-walled, are produced during sexual reproduction. Thick-walled oocysts are excreted from the host into the environment, whereas thin-walled oocysts are involved in the internal autoinfective cycle and are not recovered from stools. Oocysts are infectious upon excretion, thus enabling direct and immediate fecal-oral transmission. Clinical Symptoms The most common symptoms of cryptosporidiosis are watery diarrhea and stomach cramps. Other symptoms may include fever, nausea, vomiting, and loss of appetite. Symptoms and severity of infection vary with the age and immune status of the host. Cryptosporidium infections are uncommonly detected in cats and dogs. Cryptosporidiosis can sometimes make dogs and cats sick, but animals with signs are atypical. In most cases, epithelial damage is minimal, but in severe cases, infection is associated with losing the ability to maintain water balance. Clinical signs are usually restricted to mild diarrhea unless the host is immunosuppressed or has another underlying condition such as viral infection or malignancy. Diagnosis Cryptosporidiosis is a diarrheal disease that is spread through contact with the stool of an infected person or animal. The disease is diagnosed by examining stool samples. Oocyst excretion is intermittent, and multiple stool samples may be needed. Diagnostic methods include: Microscopic examination: Typically, stool samples are analyzed microscopically using various techniques, including acid-fast staining and Ziehl-Nielsen staining. Real-time PCR: The most accurate method for detecting Cryptosporidium spp. is through a fecal PCR assay. Immunologic tests: These include direct fluorescent antibody tests and enzyme immunoassays to detect Cryptosporidium sp. antigens. Treatment and Prevention Most patients with healthy immune systems will recover from cryptosporidiosis without treatment. Supportive measures, oral or parenteral rehydration, and hyperalimentation may be needed for immunocompromised patients with severe disease. The best way to prevent the spread of Cryptosporidium at home is by practicing good hygiene. References Sardinha-Silva A, Alves-Ferreira EVC, Grigg ME. Intestinal immune responses to commensal and pathogenic protozoa. Front Immunol. 2022 Sep 16;13:963723. Sponseller JK, Griffiths JK, Tzipori S. The evolution of respiratory Cryptosporidiosis: evidence for transmission by inhalation. Clin Microbiol Rev. 2014 Jul;27(3):575-86. Watier-Grillot S, Costa D, Petit C, et al. Cryptosporidiosis outbreaks linked to the public water supply in a military camp, France. PLoS Negl Trop Dis. 2022 Sep 12;16(9):e0010776.
Ferret Coronavirus

Bioguard Corporation The coronavirus of ferrets was first described in 1993. This ferret enteric coronavirus (FRECV) caused an enteric disease called epizootic catarrhal enteritis (ECE) or green slime disease. Later, a ferret systemic coronavirus (FRSCV)-associated disease, resembling the dry form of feline infectious peritonitis (FIP), was identified. ECE is characterized by high morbidity (nearly 100%) but low mortality (< 5%). FRSCV is associated with a high mortality rate and short duration of illness. Pathogen Coronaviruses are large, enveloped, positive-stranded RNA viruses classified under the genus Coronavirus within the family Coronaviridae, order Nidovirales. Virions are roughly spherical and are notable for the large spike (S) glycoprotein that extends from the virus envelope, which resembles a crown or solar corona when imaged using an electron microscope. The diameter of ferret coronavirus is about 120 nanometers, and its genome is about 28 kilobases. Transmission Both the enteric (FRECV) and systemic (FRSCV) ferret coronaviruses are classified as group 1 coronaviruses (that is, alphacoronaviruses), related to feline coronavirus and canine enteric coronavirus. The routes for transmission of FRECV and the FRSCV are suggested to be fecal–oral. Many facts of the pathogenesis of the virulent systemic ferret coronavirus remain unknown, but similar to FIPV, macrophages appear to play an important role in the inflammatory response. Clinical signs After infection of FRECV, the virus causes blunting of the intestinal villi and consequent maldigestion and malabsorption. Clinical signs include anorexia, vomiting, diarrhea, melena, dehydration, lethargy, and weight loss. The microscopic lesions include diffuse lymphocytic enteritis, with villus atrophy, fusion, and blunting; vacuolar degeneration and necrosis of the apical epithelium; or a combination of all these lesions. FRSCV-associated disease causing FIP-like lesions has been reported in mostly young (<18 months) ferrets. Clinical signs are nonspecific, including anorexia, weight loss, diarrhea, and enlarged intra-abdominal and, less commonly, peripheral lymph nodes. FRSCV is grossly associated with pale to white nodules (granulomatous inflammation) in multiple organs, including the spleen, kidneys, mesenteric lymph nodes, intestines, liver, lungs, and brains. Granulomas have a heterogenous cellular composition including macrophages, T and B lymphocytes, and plasma cells. Diagnosis Definitive diagnosis requires intestinal biopsy and histopathological examination, with confirmation of viral antigen or nucleic acid by immunohistochemistry or in situ hybridization. RT-PCR and electron microscopy can be used to examine feces from ferrets. Systemic coronavirus disease in ferrets can be confirmed by histological evaluation of biopsies and intralesional coronavirus nucleic acid detection. RT-PCR has been used to identify viruses in tissues and can differentiate ferret enteric coronaviruses from ferret systemic coronaviruses. References 1. Haake C, Cook S, Pusterla N, et al. Coronavirus Infections in Companion Animals: Virology, Epidemiology, Clinical and Pathologic Features. Viruses 2020, 12(9), 1023. 2. Osborne AJ, Hussain SS, Helman EE, et al. Ferret Systemic Coronavirus in Alpha-1 Antitrypsin Knockout Ferrets. Comp Med. 2022 Dec 1;72(6):410-415. 3. Gnirs K, Quinton JF, Dally C, et al. Cerebral pyogranuloma associated with systemic coronavirus infection in a ferret. J Small Anim Pract. 2016 Jan;57(1):36-9.
Infectious Myxomatosis of Rabbits
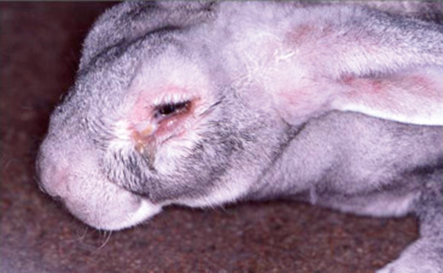
Bioguard Corporation Myxomatosis is primarily a disease of rabbits caused by infection with the myxoma virus. It mainly occurs in domestic and wild rabbits. The virus is harmless to humans. Myxomatosis can result in lumps developing around the ears and face. These lumps are named myxomas and the disease virus was named after this lesion. It was first discovered in South America, California and Mexico in 1896. Sick animals will die within a few days to two weeks after infection, and the fatality rate is close to 100%. Currently, there is no effective treatment. Pathogen Myxoma virus is the type species of the Leporipoxviruses, a genus of Chordopoxvirinae, double stranded DNA viruses, whose members infect leporids and squirrels, inducing cutaneous fibromas from which virus is mechanically transmitted by biting arthropods. Pathogenesis studies confirm that the virus initially replicates in dermal cells at the inoculation site, likely dendritic cells. From there, the virus spreads to local macrophages and epidermal cells, and to the draining lymph node. Virus replication in the latter results in lymphoid depletion, with extensive loss of cortical and paracortical lymphocytes. From the lymph node the virus spreads via blood leukocytes to distal tissues including the spleen and other lymphoid tissues, testis, lungs, and skin. Transmission and Clinical Signs Currently, myxoma virus is enzootic to the Americas, Europe, Australia and other regions. The principal mode of transmission of the virus is mechanical transport of virus on mouth parts by arthropod vectors such as ticks, mosquitoes, and fleas through bites. It can also transmit the virus to other rabbits via direct contact. The general incubation period is 3-7 days, up to 14 days. The first sign of disease is conjunctivitis that rapidly becomes more severe and is accompanied by a milky discharge from the eye. The rabbit has no energy and no appetite, with a fever that may reach 42°C. In severe outbreaks, some rabbits die within 48 hours after signs appear. Those that survive become progressively weaker and develop a rough coat. The eyelids, nose, lips, and ears become puffy, which gives a swollen appearance to the head. The ears may droop. In females, the vulva becomes inflamed and swollen with fluid; in males, the scrotum swells. Other signs include discharge of pus from the nose, difficulty breathing, and coma. Death usually occurs within 1 to 2 weeks after signs appear, and the fatality rate is close to 100%. Diagnosis Gross lesions: The most prominent gross lesions in in rabbits with myxomatosis are the skin tumors and the pronounced cutaneous and subcutaneous edema, particularly in the area of the face and around body orifices. Hemorrhages of the skin, heart, and subserosa of the gastrointestinal tract may be observed. Microscopic lesions: Lesions in the skin involve epithelial cells, fibroblasts, and endothelial cells and range from proliferative to degenerative, depending on the strain of virus. The skin tumors result from proliferation of undifferentiated mesenchymal cells, which become large stellate (myxoma) cells surrounded by a homogeneous matrix of mucinous material interspersed with capillaries and inflammatory cells Lab tests include serology and molecular diagnosis. Serology: immunofluorescent assay (IFA), ELISA, complement fixation test (CFT) Molecular diagnosis: PCR Treatment and Vaccination Unfortunately, there is no specific treatment for myxomatosis. Vet can only offer supportive care, including fluids, antibiotics to prevent secondary infections, and pain medication. The myxomatosis vaccine is available in some countries. Reference Bertagnoli S, Marchandeau S. Myxomatosis. Rev Sci Tech. 2015 Aug;34(2):549-56. Espinosa J, Ferreras MC, Benavides J, et al. Causes of Mortality and Disease in Rabbits and Hares: A Retrospective Study. Animals (Basel). 2020 Jan 17;10(1):158.
Diagnosis of Feline Respiratory Mycoplasma Infection

Bioguard Corporation In cats, ’mucosal’ mycoplasma infections typically cause ocular and respiratory disease, and less frequently neurological or joint disease. These Mycoplasma species are distinct to the haemotropic mycoplasmas that target red blood cells, causing hemolytic anemia in cats. Mycoplasma felis is typically associated with Upper Respiratory Tract Disease (URTD) in cats. Transmission M. felis is mainly transmitted from an infected cat to an in-contact one by aerosol, but also by grooming. Stresses, including overcrowding environments, concurrent respiratory viral infections, and poor hygienic situations, may promote transmission of the infection between cats. Clinical symptoms Mycoplasma felis is typically associated with URTD but sometimes it may be associated with lower respiratory tract infections. Common clinical signs include clear or colored discharge from the eyes or nose, coughing, sneezing, conjunctivitis, chemosis, lethargy, and anorexia. Lower respiratory tract infections can result in pneumonia with fever, cough, tachypnoea, and lethargy. Diagnosis Culture of mycoplasmas can be used to demonstrate infection, but it takes time for culture and rapid transport of samples to the laboratory is required. Demonstration of organisms via real-time PCR is increasingly being used to circumvent the difficulties with culture, Treatment Antimicrobial therapy is commonly used to treat mycoplasma respiratory infections. Doxycycline is a good first line agent because it is well tolerated by cats and relatively narrow in spectrum. The recommended dose is 5 mg/kg, PO, q12h or 10 mg/kg, PO, q24 (Lappin et al., 2017). Oxytetracycline or chlortetracycline ophthalmic ointment can be used q6h in addition as topical treatment. References: Vekšins A. Feline upper respiratory tract disease – Computed tomography and laboratory diagnostic. Vet World. 2022 Jul;15(7):1880-1886. Framst I, Ramesh P, Cai HY, Maboni G. Complete genome sequences of Mycoplasma cynos and Mycoplasma felis isolated from dogs and cats with infectious respiratory disease. Microbiol Resour Announc. 2024 Apr 11;13(4):e0124323.
Tritrichomonas Infection in Cat
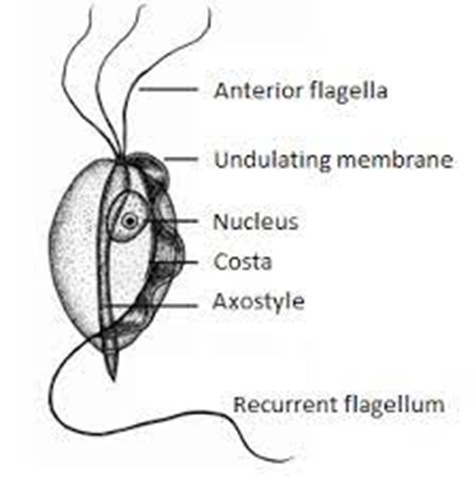
Bioguard Corporation Tritrichomonas foetus is a significant cause of large bowel diarrhea and persistent colitis in cats. These pear-shaped organisms have three anterior flagella and one posterior flagellum. They have a distinctive undulating membrane, which gives them a similar appearance to Giardia. However, they do not form cysts and are transmitted directly from one host to another as trophozoites. The infection is most prevalent among young cats living in close quarters, such as in densely populated catteries and shelters. A notable investigation into purebred show cats discovered a 31% infection rate among 117 cats spanning 89 catteries, as detailed in Gookin’s 2004 study Clinical Signs Some cats infected with T. foetus may not exhibit any symptoms, particularly older cats that are in good health. However, most cats with T. foetus infection suffer from mild to severe lymphoplasmacytic and neutrophilic colitis, which causes recurrent episodes of large bowel diarrhea that may vary in consistency from semiformed to “cow pie” and emit a foul odor. Diarrhea may contain fresh blood or mucus. Insevere cases, kittens may experience painful anal irritation, fecalincontinence, or even rectal prolapse. Affected cats usually maintain a healthy appearance and good body condition overall. The presence of diarrhea can be exacerbated by other intestinal infections or parasites, particularly Giardia and ryptosporidium. Diagnosis To confirm the infection of T. foetus, there are three methods available – direct fecal microscopy, fecal culture, and fecal polymerase chain reaction (PCR) assay. Direct fecal microscopy involves identifying the motile trophozoites T. foetus in fresh wet smears of diarrheic feces taken directly from the rectum. This method identifies the organisms in about 14% of cases and is less effective with formed or dried feces. In cats who have been treated with antibiotics recently, the detection rate decreases. Trichomonads, which resemble Giardia in size and shape, can be distinguished by their unique undulating membrane and rapid, jerky motility, contrasting with Giardia’s “falling leaf” movement. Fecal culture can be done in-house or at a specialized lab. It helps increase the chances of identifying the organisms. In specific cases, a saline flush might be performed by inserting a catheter through the cat’s anus to wash the colon with saline, followed by aspiration of fecal material. Fecal PCR is the most accurate method for identifying T. foetus. To perform this test, the fecal sample should not contain any litter. This technique detects the organism’s DNA traces in the cat’s stool. It’s best to conduct testing on cats that have not received antibiotics for at least two weeks for the most accurate test results. Antibiotics can temporarily reduce the number of T. foetus, leading to false negatives. Treatment Often, many approaches for treating chronic diarrhea have been tried unsuccessfully before a true diagnosis of T. foetus is confirmed. Tritrichomonas foetus is resistant to most antibiotics and is extremely difficult to eradicate (Gookin 2001). Numerous antibiotics have been evaluated. Some antibiotics reduce the number of organisms and improve the diarrhea without eliminating the infection, so diarrhea relapses whenever antibiotics are stopped. Diarrhea is typically refractory to corticosteroids. The most successful treatment for eliminating T. foetus is ronidazole (30 mg/kg PO, once or twice daily for 14 days). The side effects in some cats include lethargy, decreased appetite, and neurotoxicity. Cats with neurotoxic signs usually improve when the drug is stopped, but recovery can take 1 to 4 weeks. Ronidazole should not be used in pregnant and nursing queens or in very young kittens. Ronidazole is not approved for veterinary or human use, but some pharmacies compound chemical grade ronidazole for veterinary use. Because of its bitter taste, ronidazole compounded in gel caps is better tolerated than flavored suspension. When prescribing ronidazole obtain informed consent and instruct owners to wear protective gloves when handling it. Management of Tritrichomonas foetus Infection In cases where cats show mild or sporadic symptoms of diarrhea caused by T. foetus, and treatment is not possible due to potential side effects, costs, or the owner’s preferences, it is important to know that diarrhea may naturally go away with time, which can take up to two years. However, such cats are likely to remain lifelong carriers of the parasite. The outlook for cats receiving treatment is generally positive. A majority of treated cats exhibit better stool consistency in just a few days, though diarrhea might linger briefly as related secondary inflammation subsides. Nonetheless, around 25% of cases might experience a continuous infection despite initial treatment. Fortunately, T. foetus has a short lifespan outside its host and is easily neutralized by common disinfectants. To mitigate infection risks, it’s advised to uphold strict litter box cleanliness through daily cleansing, isolate cats under treatment, minimize stress factors, prevent overcrowding, and implement regular screenings in breeding and shelter settings whenever feasible.
Encephalitozoon cuniculi in Rabbits

Bioguard Corporation Encephalitozoon cuniculi is a microsporidial, unicellular, spore-forming, obligate intracellular parasite. It can invade the host’s central nervous system, kidneys, crystals, etc. E. cuniculi affects rabbits by causing damage to the brain, nervous system, kidneys, and other important organs. E. cuniculi pose a zoonotic risk to immune-compromised humans. In addition, it can infect various mammals, such as rabbits, rats, mice, horses, foxes, cats, dogs, muskrats, leopards, and baboons. Transmission and Life Cycle E. cuniculi has a direct life cycle with both horizontal and vertical (transplacental) transmission. In rabbits, the common routes of natural horizontal infection are via the ingestion of contaminated food or water or, less commonly, via inhalation of spores. After ingestion, the spores invade enterocytes and then spread through bloodstream or the lymphatic system. Then, it is carried into the blood circulation to target organs (kidney, central nervous system, eye, liver, and heart) where it causes inflammation. Antibody can be detected 2-3 weeks after infection, and IgM are usually detectable up to 18 weeks post-exposure. Spores are passed in the urine of rabbits, beginning around 35 days after infection, and continue to be excreted for 2 to 3 months. Serological surveys show high seroprevalence rates (23-75%) of E. cuniculi in rabbits. The seroprevalence rate of E. cuniculi is about 63.2-67.8% in Taiwan. However, most are asymptomatic and few are found to be affected by disease. Clinical signs When E. cuniculi infect the rabbits, the common clinical signs include head tilt (vestibular disease), hind limb paresis (weakness of the hind limbs), urinary incontinence, renal failure, cloudy eyes (anterior uveitis), cloudy lenses of the eyes (cataracts), or even blindness. Diagnosis Clinical diagnosis of encephalitozoonosis can be challenging because of the following reasons. 1. Serologic evidence is strong evidence of infection but not indicative of clinical signs. 2. Seroconversion does not result in a protective response for the patient. 3. Histologic severity and distribution of lesions are not directly correlated with the severity of clinical signs. 4. Most infected rabbits are asymptomatic or carriers. Diagnostic methods of encephalitozoonosis include histopathology, serology, and molecular diagnosis. • Histopathology- histological examination combined with special staining, or concentrated urine for cytological microsporidia detection • Serology- indirect immune fluorescence antibody test, direct agglutination test, ELISA, western blot • Molecular diagnosis- PCR Treatment and Prevention No uniformly effective treatment has been established. Fenbendazole, Albendazole, and Oxibendazole may be effective in vivo. The treatment of choice generally is fenbendazole, because it has been shown to both prevent and treat E cuniculi infections. To reduce the inflammatory reaction, steroids or NSAIDS may be applied. The use of systemic steroid therapy, although controversial, has been advocated to reduce severe CNS inflammation. The risk with this treatment is that corticosteroids might suppress the immune system to the point that E cuniculi or other infectious organisms may create additional problems. Cases involving the ocular form need to be referred to a veterinary ophthalmologist if surgery is recommended. Prevention of the disease from spreading includes thoroughly cleaning the rabbit’s environment, applying a diluted solution of bleach (1:32) for disinfection, and providing clean water and food to rabbits.
Psittacosis in Birds
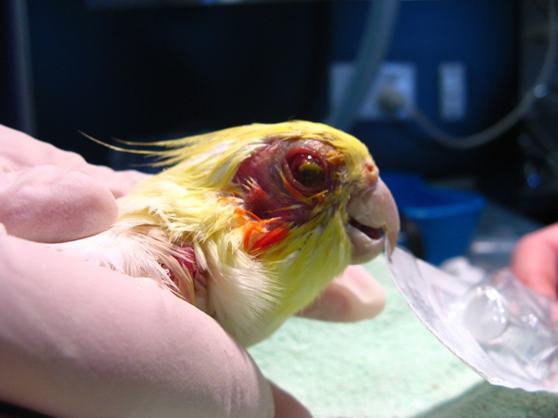
Bioguard Corporation Psittacosis (parrot fever), also known as ornithosis, is a bacterial zoonotic disease caused by the bacterium Chlamydia psittaci. It is usually spread by exposure to infected birds at home, pet stores, pigeon stalls and other locations where birds are kept or displayed. The disease was first reported in Switzerland in 1879, and then occurred successively in Britain, Europe, USA, Central and South America and other regions. The incubation period varies from 5-28 days. The clinical symptoms are nonspecific and may include fever, chills, headache, muscle aches, and cough. Etiology Chlamydia psittaci includes six avian (A–F) and two mammalians (WC in cattle and M56 in muskrat) serotypes with distinct host specificity, based on serotyping using monoclonal antibodies in the 1990s. Different serotypes have been isolated from different avian species like serotype A in psittacine birds, B and C in both ducks and geese, D in turkeys, E mainly in pigeons (also in other avian species), and F in parakeets and turkeys. Serotype A is often reported in human zoonotic cases. Based on recent genotypic classification, C. psittaci has several classical genotypes with relevant host specificity. The genomic sequence encoding the outer membrane protein (ompA) is used to classify genotypes, which are employed to study isolates from infected birds and isolates in mammals. All identified genotypes are considered capable of transmission to humans. Transmission Infected birds may shed C psittaci in their droppings, saliva, mucus, feather dust and eye/nasal discharge. Healthy birds may then inhale the bacteria in airborne particles, such as dust from dried droppings and feathers. Birds may also ingest C psittaci from contaminated food, water, perches, and toys. Some infected birds don’t show any signof illness. However, they may become ill and shed the bacteria during times of stress (e.g., battling another infection, changing diet, or living in a crowded environment). Humans most commonly catch the disease from infected birds by breathing in the dust from shed feathers, secretions, and droppings. Less commonly, birds infect people through bites and beak-to-mouth contact. Clinical symptoms The incubation time for infected birds is about 3 days to several weeks up to a month. In birds, the symptoms include poor appetite, ruffled appearance, eye or nose discharge, green or yellow-green droppings, and diarrhea (loose droppings). Occasionally, birds may die from the disease. Some birds may shed the bacteria while exhibiting only mild or no symptoms. C. psittaci may affect some or all of a bird’s organ systems, most commonly the liver, spleen, respiratory tract, and digestive tract. Diagnosis Since psittacosis symptoms can look like an array of other diseases in birds, special tests are needed to diagnose the presence of C. psittaci. Methods to diagnose chlamydial infections include: Antigen detection- immunohistochemistry, immunofluorescence test and ELISA Serology- complement fixation, ELISAs, and indirect immunofluorescence test Molecular diagnosis- PCR Treatment Treatment is usually with oral or injectable doxycycline antibiotic. Since doxycycline only kills the Chlamydia organisms when they are active and dividing, and the lifecycle of these organisms is prolonged, with possible periods of dormancy (ceasing to be active for a period of time), drug treatment should go on continuously for the recommended period of 45 days, without interruption.



