Case study: Report of the first clinical case of intestinal trichomoniasis caused by Tritrichomonas foetus in a cat with chronic diarrhoea in Brazil
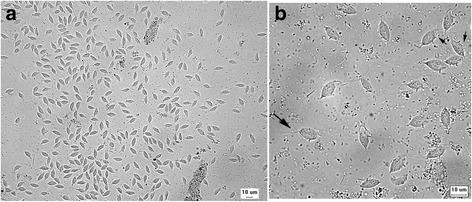
Case study: Report of the first clinical case of intestinal trichomoniasis caused by Tritrichomonas foetus in a cat with chronic diarrhoea in Brazil Robert Lo, Ph.D, D.V.M A seven-month-old, entire male domestic shorthair kitten was presented to the Veterinary Hospital of the School of Veterinary Medicine – University of São Paulo, Brazil. The cat showed a six-month history of persistent large intestinal diarrhoea, faecal incontinence, prostration, apathy and weight loss. P Protozoan parasites were observed under microscope using fresh fecal sample obtained via colon flush. Infection of Tritrichomonas foetus was confirmed by PCR and DNA sequencing. After treatment with ronidazole (30 mg/kg, PO q24h for 14 days), the cat showed resolution of clinical signs. This is the first clinical case of T. foetus infection in a chronic diarrheic cat in Brazil and South America. Original paper: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5392982/ Fig. 1 Tritrichomonas foetus in cat feces. a Numerous pyriform trophozoites. b The three free anterior flagella (large arrow) and the undulating membrane (small arrows) can be visualised in some trophozoites. Fresh preparation in saline 0.85%.
Breed-related disease: Beagle

John K. Rosembert The beagle is a breed of small hound that is similar in appearance to the much larger foxhound. The beagle is a scent hound, developed primarily for hunting hare (beagling). Possessing a great sense of smell and superior tracking instincts, the beagle is the primary breed used as detection dogs for prohibited agricultural imports and foodstuffs in quarantine around the world. Overall, Beagles are small, hardy hounds. They have a short coat, a deep chest, stocky legs, and a medium-length tail. Their heads are long compared to their bodies, with low-set drooping ears. Big brown or hazel eyes are set well apart and gaze with the typical, soft hound expression. Their standard coat is tricolored with white, black, and brown. It is a loving, sweet, and gentle, happy to see everyone by greeting them with a wagging tail. It is sociable, brave, and intelligent. The Beagle is excellent with children and generally good with other dogs, but because of its hunting instincts, it should not be trusted with non-canine pets, unless socialized with cats and other household animals when young. Beagles have minds of their own. They are determined and watchful and require patient, firm training. If you’re wondering about what common health problems affect this breed, below we put some of the most common health conditions that may affect this breed. Back Problems: Intervertebral disc disease (IVDD) is a common condition in Beagles. The disease is caused when the jelly-like cushion between one or more vertebrae slips or ruptures, causing the disc to press on the spinal cord. If your dog is suddenly unable or unwilling to jump up, go upstairs, is reluctant to move around, has a hunched back, cries out, or refuses to eat or go potty, he is likely in severe pain. He may even drag his back feet or be suddenly paralyzed and unable to get up or use his back legs. If you see symptoms, don’t wait. Call an emergency clinic immediately! Eye problem: Beagles are prone to numerous eye conditions. These can range from small eyes (microphthalmia) to cataracts, glaucoma, and retinal problems called progressive retinal atrophy. They also get a prolapse of the third eyelid gland (cherry eye), which appears as a red membrane over the eye. Any time your pet has eye discharge, redness, or is pawing at one of its eyes, and examination should be performed. Bleeding disorder: On occasion, some Beagles can get a bleeding disorder. If your pet easily bruises or seems to take a long time to clot blood, it should have an examination. Beagles are susceptible to herniated discs. Any time it shows signs of pain or inability to walk properly, this disease might be a cause. Epilepsy: is another relatively common problem in Beagles, this disease manifests itself as a seizure. Any time your pet has a seizure it should be brought to our attention. Heart disease: Beagles are prone to multiple types of heart disease. Symptoms, if they occur, include distended abdomen, difficulty breathing, exercise intolerance, and unfortunately, even sudden death. Hyperadrenocorticism (Cushing’s): Cushing’s disease is a malfunction of the adrenal glands causing them to produce too much steroid hormone. This is a common problem in dogs, and Beagles are more likely than other dogs to be affected. The condition usually develops slowly, and the early signs are easily missed. Symptoms include drinking and urinating more than normal, increased appetite, and reduced activity level. Later, a potbelly, thin skin, and hair loss are characteristic. Treatment usually includes oral medications and requires close coordination to ensure correct dosing. https://www.petcoach.co/breed/beagle https://www.dogbreedinfo.com/beagle.htm https://animalhealthcenternh.com/client-resources/breed-info/beagle/ https://www.lbah.com/breed-disease/beagle-diseases/ Photo credit: https://www.doggies.com/Beagle https://www.animaroo.com/dog-breeds/beagles.html
Breed-related disease: Bengal cat

John K. Rosembert The Bengal cat is a domesticated cat breed created from hybrids of domestic cats, especially the spotted Egyptian Mau, with the Asian leopard cat, the breed name comes from the leopard cat’s taxonomic name. It is a long, muscular, medium- to large-sized cat, with a broad head and muzzle, high cheekbones, and pronounced whisker pads. The eyes are round and wide, with dark markings around the eyes (mascara) and the ears small and rounded at the tips. The grace of a jungle cat is held as one of the positive characteristics, along with the ability to move quietly and with stealth. The Bengal cat is known for its soft, sleek coat which has two main fur patterns: spotted (which is most common) and marbled. Both patterns are often tri-colored, giving each cat unique markings and patterns. This tri-coloring gives some Bengals spots which have a darker outline, often like the spots on a Jaguar. Because the Bengal is a hybrid developed from crossing the Asian Leopard cat and the American Shorthair. The result was a cat with an exotic look and a domestic temperament. The intelligent and athletic Bengal is an entertaining companion that demands human contact and can be very vocal in their pursuit of attention. The Bengal can be aggressive with other cats and needs to be socialized at an early age. They are highly active and agile, usually in constant motion. Bengals enjoy climbing, jumping, and a good game of fetch. Be sure to provide them with plenty of toys and a tall climbing tree to keep them amused and out of trouble. Below we will summarize some of the most major concerns of Bengal cat in order to help you prevent some predictable risks in your pet. Noted that before purchasing or adopting a Bengal, make sure the breeder offers a health guarantee on the kittens. Here are some of the most common diseases related to Bengal cat Joint problems: More often found in small dogs, Bengal cats may experience luxating patellas. luxating patella is a kneecap that slips off to the side of the leg because of an improperly developed stifle. A cat with a luxating patella may not show signs of pain or abnormality until the condition is well advanced; signs of this condition appear gradually and can progress to lameness as the cat grows older. Hip Dysplasia: another disease most commonly found in dogs, hip dysplasia may also occur in cats, especially in Bengals. Dysplasia is an inheritable condition that causes malformation of the hip joints and subsequent arthritis. Anesthetic Allergies: if your Bengal is going in for any type of surgery, including spaying and neutering, your vet must be careful regarding the use of anesthetics. Bengals, extremely sensitive to anesthetics, may experience allergic reactions that cause cardiac arrest. Always discuss the type of anesthetic with your vet before any surgery. Heart Disease: A heart condition, hypertrophic cardiomyopathy, is common in Bengals. This disease of the heart muscle usually occurs in the older cat. The heart muscle thickens, so the organ must work much harder, causing a number of problems. These may include blood clots, or thrombosis, rendering the back legs immobile. The disease also leads to congestive heart failure, resulting in death. Early signs of cardiomyopathy include panting and lethargy. https://www.petmd.com/cat/breeds/c_ct_bengal https://pets.thenest.com/bengal-cat-health-problems-4479.html https://animalhealthcenternh.com/client-resources/breed-info/bengal/ Photo credit: https://cattime.com/cat-breeds/bengal-cats
Feline Pancreatic Lipase
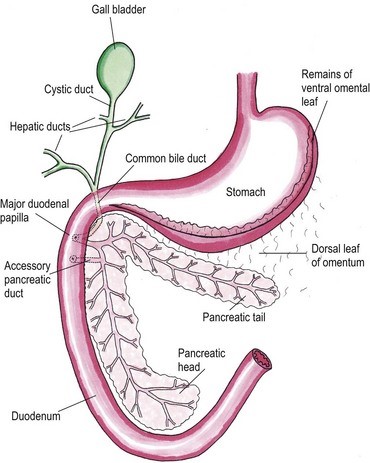
Feline Pancreatic Lipase LIN, WEN-YANG (WESLEY), Ph.D Feline Pancreatic Lipase is a powerful diagnosing biomarker for feline pancreatitis, whereas serum amylase and serum lipase are usually used in diagnosing pancreatitis for canine, are not effectively in detecting pancreatitis for cats. Anatomical physiology function of pancreas in cats The feline pancreas is a digestive glandular organ, which presents a long v-shaped strip of configuration locating at abdomen between stomach and duodenum. The tail part of feline pancreas rests toward the dorsal extremity of the spleen and connect to mesocolon with omentum (Figure 1). Figure 1. Anatomic view of the pancreas and its surrounding tissues https://veteriankey.com/pancreas-4/ The normal pancreas colored on pale pink and perform both functions of endocrine and exocrine. The endocrine portion contains small clusters of pancreatic α- and β-cells within Langerhans (approximately 2% of the gland’s weight) that majorly generates two hormones proteins: glucagon and insulin. Glucagon works to elevate the level of blood sugar, whereas insulin would diminish excess circulating blood sugar. Both of glucagon and insulin would take part in regulating homeostasis of glycemic level. Acinar and ductal cells are major members in the exocrine portion of pancreas. Acinar cells aggregate around the terminal pancreatic ductules to secrete digestive enzymes include trypsinogen, chymotrypsinogen, proelastase, procarboxypeptidase, ribonucleases, deoxyribonucleases, phospholipase A2, carboxylesterase amylase and lipase. Amylase and lipase are contributing to hydrolyze carbohydrates and fat. Others may play roles as proteases to cleave polypeptide chains. Feline’s ductal cell generates antibacterial proteins to protect small intestinal from bacterial infection. Moreover, ductal cell can also produce bicarbonate and water for neutralizing pH in the duodenum. Intrinsic factor such as vitamin B12 would be made from ductal cell too. The pancreas is the only generating organ of intrinsic factor for feline, whereas dogs could produce intrinsic factor from pancreas and stomach. All pancreatic enzymes would secret into small intestine for digesting fats, proteins and carbohydrates. Furthermore, the abnormal seep of exocrine enzymes to pancreas and its surrounding organs could cause pancreatic inflammation known as pancreatitis. The highest occurrence of feline pancreatic diseases is exocrine pancreatic insufficiency (EPI) and pancreatitis. Pancreatitis in the cat Feline pancreatitis is classified as acute (temporary morphological changes) and chronic (permanent morphological change) type according to the condition of histopathologic changes after treatment. Acute necrotizing pancreatitis (ANP) and acute suppurative pancreatitis are two of the most common types of acute feline pancreatitis, whereas chronic non-suppurative pancreatitis (CP) and pancreatic atrophy are chronic conditions. Common acute pancreatitis in feline would show anemia, leukocytosis, hypokalemia, hypocalcemia, hyperglycemia, elevation of ALT, ALP, total bilirubin, cholesterol and decreasing level of albumin. Acute necrotizing pancreatitis (ANP) usually present pancreatic acinar cell necrosis, peripancreatic fat necrosis followed inflammation, hemorrhage, mineralization and fibrosis. Despite of its idiopathic character, several diseases have considered to be related with development of ANP including concurrent biliary tract disease, ischemia, pancreatic ductal obstruction, toxoplasmosis, feline Herpes virus infections, feline infectious peritonitis, pancreatic fluke infestations (Eurytrema procyonis, Amphimerus pseudofelinus), trauma, organophosphate poisoning and hepatic lipidosis. General anesthesia induced hypotension or surgical venous outflow occlusion would decrease pancreatic blood flow and cause ANP. Acute suppurative pancreatitis is less common than ANP in feline and neutrophilic inflammation would happen with it. Besides, the continuous and progressive inflammatory process of the pancreas would lead to chronic non-suppurative pancreatitis (CP) in which lymphocytic inflammation, fibrosis, and acinar atrophy are the major features. The end stage of CP in most feline cases would usually result in pancreatic atrophy, which may or may not influence the endocrine portion of the gland. Felines who suffer pancreatic atrophy would occur cobalamin and fat-soluble vitamin malabsorption, severe maldigestion, acid injury in duodenal mucosa, and bacterial proliferation in the gut due to exocrine pancreatic insufficiency. Since various types of pancreatitis such as acute and chronic pancreatitis, pancreatic abscess, pancreatic cyst/pseudocyst, exocrine pancreatic insufficiency, and neoplasia share overlapping symptoms, thus histopathology cab be used to discern different conditions. Symptoms of feline pancreatitis Symptoms of feline pancreatitis would occur lethargy, anorexia, dehydration, hypothermia, vomiting, weight loss, Jaundice, cholangiohepatitis, hepatic lipidosis, biliary obstructions, cranial abdominal masses and cranial abdominal discomfort. Besides, hepatic and intestinal disease would concurrent. I. Acute feline pancreatitis: Vomiting, poor appetite, poor activity, diarrhea, abdominal pain, drooling, fever, collapse. II. Chronic feline pancreatitis: Frequent vomiting, poor appetite, listless, frequent diarrhea, abdominal pain, drooling, fever, collapse, hypothermia, breathing too fast or too slow, fast heartbeat. Possible Causes of feline pancreatitis Scientists presumed that premature trypsin activate of digestive zymogens in pancreatic acinar cells would cause pancreatic autodigestion, acinar cell necrosis, hemorrhage, and fat necrosis, saponification, mast cell degranulation, leukocyte chemotaxis, platelet aggregation, vasodilation, surfactant degradation within the lungs and initiation of disseminated intravascular coagulation (DIC) for worse cases. Diagnosis and general considerations Since symptoms of feline pancreatitis were similar to common flu, vets probably can’t judge it correctly at the early stage without proper diagnostic tools. It’s not an easy task to diagnose pancreatic disease within cats. Single diagnostic method is not recommended. The diagnosis of feline pancreatitis relied on combinational diagnostic tools include historical data, physical examination results, laboratory, and Bio-imaging. Especially, histopathology plays the role as the definitive conclusion for feline pancreatitis. Middle-aged felines are susceptible to pancreatitis; whereas, older felines (mean 12.8 years, range 4–20 years) are susceptible to neoplasia, cyst/pseudocysts. Diagnostic imaging Radiography, computed tomography (CT) and ultrasonography were effective imaging tools applied in diagnosing feline pancreatitis. Among all imaging tools, ultrasonography is the most reliable imaging modality for the diagnosis of feline pancreatic diseases, which can help identify soft tissue masses, cysts/pseudocysts, abscesses, or neoplasia and lesions of feline pancreatitis. Clinicopathologic tests The general clinicopathologic test for feline pancreatitis include complete blood cell count, serum bilirubin, cholesterol, glucose, total protein, albumin, and serum activity of liver enzymes (serum alanine aminotransferase and alkaline phosphatase), calcium, urea, creatinine, and potassium. However, serum lipase and amylase activities can’t be diagnostic indicator for feline pancreatic disease. Nevertheless, feline trypsin-like immunoreactivity (fTLI) and
Feline Alpha-1-acid glycoprotein (AGP)
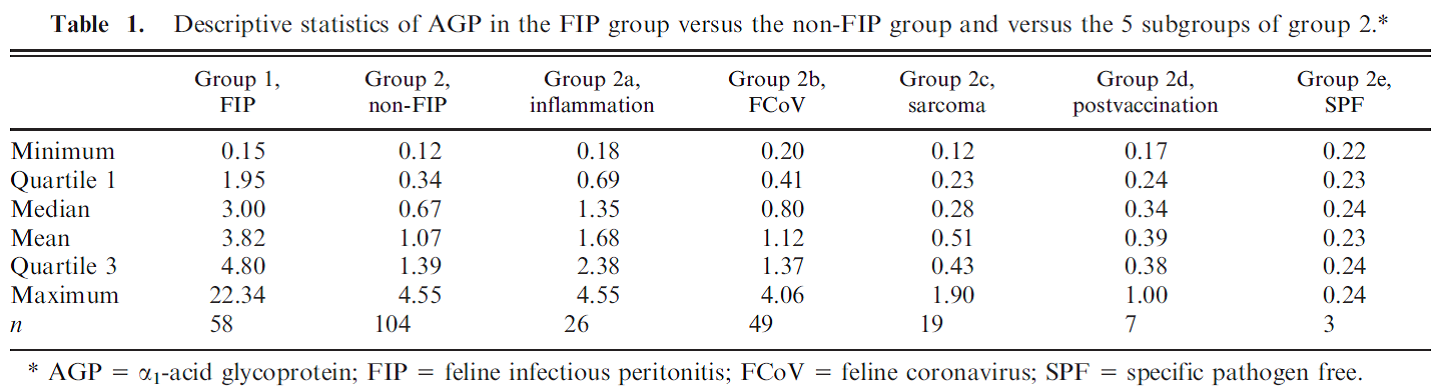
Feline Alpha-1-acid glycoprotein (AGP) Andy Pachikerl, Ph.D Introduction Alpha-1-acid glycoprotein (AGP) surges in cats’ blood when they fall in victim of feline infectious peritonitis (FIP), a lethal disease caused by feline coronavirus (FCoV). The diagnosis of feline infectious peritonitis (FIP) is often tough and not very viable at times. The clinical suspicion of FIP might be supported by the detection of effusions if any are present (Hartmann, et al., 2003; Paltrinieri, Parodi, & Cammarata, In Vivo Diagnosis of Feline Infectious Peritonitis by Comparison of Protein Content, Cytology, and Direct Immunofluorescence Test on Peritoneal and Pleural Effusions, 1999). The only way to conclusively test FIP to be positive is through histology followed by immunohistochemical or immunofluorescent detection of feline coronavirus (FCoV) within intralesional macrophages (Addie, Paltrinieri, & Pedersen, 2004; Barlough & Stoddart, 1990). Various studies suggested the implementation of biopsies for the confirmation of FIP in veterinary practices and it turns out to be quite useful with just a few downside (Alessia, Paltrinieri, Bertazzolo, Milesi, & Parodi, 2005). The application of biopsies in vivo is usually restricted due to anaesthetic risks especially via surgical biopsy and the relatively high percentage of unsuitable or falsely negative tru-cut or fine-needle aspiration biopsies (Alessia, Paltrinieri, Bertazzolo, Milesi, & Parodi, 2005). Serology and polymerase chain reaction techniques are not suitable for FIP diagnosis because they do not differentiate between the widespread low pathogenic FCoVs and the mutant pathogenic FCoV strains (Addie, Paltrinieri, & Pedersen, 2004; Herrewegh, et al., 1997). A previous study (Stoddart, Whicher, & Harbour, 1988) reported high levels of a1-acid glycoprotein (AGP) in cats with experimentally induced FIP. This finding was confirmed by another study, (Duthie, Eckersall, Addie, Lawrence, & Jarrett, 1997) which proposed the possible use of serum AGP as a diagnostic tool for FIP. Serum AGP is now widely used in diagnostic profiles for FIP.1 However, serum AGP levels increase in inflammatory disorders other than FIP (Duthie, Eckersall, Addie, Lawrence, & Jarrett, 1997; Kajikawa, Furuta, Onishi, Tajima, & Sugii, 1999; TerWee, Lauritzen, Sabara, Dreier, & Kokjohn, 1997; TerWee, et al., 1998), neoplasia (Correa, Mauldin, Mauldin, & Mooney, 2001), and asymptomatic but FCoV-positive cats. This lack of specificity limits the diagnostic potential of serum AGP as a diagnostic test for FIP. For more information on FIP and FCoV can be found in previously published report article (link). FIP clinical diagnosis through feline AGP AGP has been used extensively, particularly in Europe, as an indicator test for FIP. AGP was found almost a decade ago to be hyposialylated in cats with FIP, but not in normal cats or in cats with other pathologies (Fabrizio, Claudia, Alessia, Vanessa, & Saverio, 2004). This study confirmed that serum AGP is a powerful discriminating marker for FIP, but only when coupled with other high risk factors (Saverio, Giordano, Tranquillo, & Guazzetti, 2007). A Bayesian approach demonstrated that, when the pre-test probability of FIP was high based on history and clinical signs, moderate serum AGP levels (1.5–2 μg / mL) could discriminate cats with FIP from others. However, only high serum AGP levels (>3 μg / mL) were highly suggestive of FIP in cats with a low pre-test probability of disease (Saverio, Giordano, Tranquillo, & Guazzetti, 2007). Giori, et al. (2011) had shown specificity and sensitivity of several tests in 12 cats, four of which have the absence of FIP via histopathology and immunohistochemistry, and eight cats with FIP confirmed via histopathology and immunohistochemistry. Results from serum protein electrophoresis, analysis of effusions, anti-feline coronavirus serology, serum AGP concentrations and histopathology were then compared with the confirmed diagnosis. No concordance was found for serology and analysis of effusions, poor concordance was noted for histopathology, fair concordance for serum electrophoresis and perfect concordance for AGP. Their study proved that immunohistochemistry is always required to confirm FIP and, if immunohistochemistry is not feasible, they concluded that histopathology is not definitive, whilst elevated AGP concentrations might support the diagnosis of FIP. However, the small numbers of cats in this study make it difficult to validate such conclusions and the earlier study of Saverio, et al. (2007) is probably a more accurate assessment of AGP testing for FIP. Like most indirect tests for FIP, the positive predictive value increases with the number of other risk factors that are present. Saverio, et al. (2007) also investigated the levels of leukocyte bound AGP in normal cats and cats with diseases including FIP by flow cytometry using an anti-feline AGP antibody. A total of 32 healthy cats (19 feline coronaviruses seropositive), 13 cats with FIP (presumably all coronavirus seropositive) and 12 cats with other diseases (six coronaviruses seropositive) were studied. The proportion of cats with AGP-positive leucocytes in each group or in cats with different intensities of inflammatory response (as measured by CBC, serum electrophoresis and serum AGP levels) was compared. AGP positive leucocytes were found in 23% of cats; most were diseased, but a small number were healthy. AGP positive leukocyte staining was associated with inflammation and not with leucocytosis per se. Staining among healthy cats was unrelated to coronavirus antibody status. Cats with FIP were more likely to have positive staining leukocytes than healthy cats, but not as likely as cats with other diseases. It was concluded that AGP positive leucocytes are present in feline blood, especially during inflammation. Staining leukocytes for AGP binding do not appear to have any value over serum AGP testing, especially when considering the potential cost and effort involved in this method. A previous study by Paltrinieri, et al. (2007) showed positive correlation between AGP and FIP cats. In their study, they used 2 different groups of cats with FIP or non-FIP along with others contracted with other diseases such as FCoV. Their schematic experiment is as follows: Group 1. FIP group. This group was composed of 58 cats that had clinical signs and laboratory findings confirmatory of effusive FIP (n 5 53) or dry FIP (n 5 5). Haematology and serum biochemistry in these cats revealed nonregenerative anaemia, neutrophilia, lymphopenia, increased total
Breed-related disease: German Shepherd

The German Shepherd is a breed of medium to large-sized working dog that originated in Germany, Intelligent as it is versatile, this breed was originally developed in Germany to guard and herd a shepherd’s flocks. It has a double coat, which is comprised of a thick undercoat and a dense, slightly wavy or straight outer coat. Its hair, usually tan and black, or red and black, is medium in length and is shed all year round. Other rarer color variations include all-Black, all-White, liver and blue. The German Shepherd’s body is long-generally between 22 and 26 inches-in proportion to its height. This gives the dog strength, agility, elasticity and long, elegant strides. Because this watchful, self-assured breed is nearly unmatched in intelligence, German Shepherds excel in high-pressure jobs that require next-level problem solving, like search and rescue or police work. These extremely confident dogs are also keen observers and thinkers who have an uncanny ability to make decisions and problem-solve on the fly. They’re lauded for their courage, which is another trait that makes them a versatile working companion. Though German Shepherds might seem aloof around strangers, they bond easily with their families and are incredibly loving companions. The German Shepherd has an average lifespan of between 10 to 12 years. It is, however, susceptible to some serious health conditions like: Perianal Fistula: which is a disorder most commonly seen in German Shepherds. The disease is characterized by draining openings on the skin around the anus. Affected dogs may strain to defecate, have diarrhea or bloody stool and lick at the anal area frequently Megaesophagus: (from the Greek Mega meaning large) is a condition in which the esophagus (the tube that carries food to the stomach when we swallow) becomes limp and is not able to normally pass the food on its way to be digested. The type of megaesophagus that we see in German Shepherds is a congenital problem that a recent study found to correlate to chromosome 12. Affected dogs often begin to show signs, vomiting and regurgitation when they are weaned to a solid diet. Hip Dysplasia: Most people by now know about hip dysplasia. The hip joint is a ball and socket joint and hip dysplasia causes malformation of the components leading to instability. There can be abnormalities in either the ball or the socket (or both) and the chronic laxity causes abnormal wear and leads to osteoarthritis. Degenerative Myelopathy : is a neurologic disease and is a recessive genetic disorder in the German Shepherd Dog. Affected dogs are usually middle-aged or older patients and this disorder are difficult to distinguish from other causes of spinal cord compromise like intervertebral disc disease found commonly in many types of dogs. This genetic cause of weakness and paraplegia can only be positively identified postmortem with a histological exam of spinal cord tissue. Exocrine Pancreatic Insufficiency (EPI): This disorder of the digestive system is potentially life-threatening (particularly in its acute form) but often responds well to treatment. It is more common in some breeds than others and is frequently seen in German Shepherd Dogs. Sources: https://www.petmd.com/dog/breeds/c_dg_german_shepherd https://iheartdogs.com/ask-a-vet-what-are-5-important-health-concerns-for-german-shepherd-dogs/ Photo credit: https://en.wikipedia.org/wiki/German_Shepherd https://www.akc.org/dog-breeds/german-shepherd-dog
Breed-related disease: Siberian cat

The Siberian is a centuries-old landrace (natural variety) of domestic cat in Russia, and recently developed as a formal breed with standards promulgated the world over since the late 1980s. As befits a cat from northern Russia, the Siberian wears a magnificent fur coat that not only protects him from the elements but also gives him a glamorous appearance that belies his gentle good nature. At first glance, the Siberian resembles the Maine Coon and the Norwegian Forest Cat, but he is differentiated by having a more rounded body and head. He also stands out for his large yellow-green eyes, tufted ears and neck ruff. The Siberian coat comes in many colors and patterns, but brown tabbies seem to be most popular. The Siberian cat is highly affectionate with family and playful when they want to be. However, their exercise needs aren’t overly demanding, and they’re just as happy to snuggle up with their humans as they are to chase a laser toy–maybe even happier. In Russia, the phrase Siberian health is associated with vitality, longevity, and ability to stay healthy despite the frigid climate of the Siberian region. This saying is very true when it comes to Siberian cats. Siberians tend to be sturdy, healthy and, while being purebred cats, do not present the owner with too many health issues. However, there are some health problems typical for cats in general, and for Siberians in particular. If you own a Siberian cat or kitten or are only planning to adopt one, it’s best to know ahead what types of health issues you may encounter, and how to help your cat overcome them. Hypertrophic Cardiomyopathy : This is a heart condition in which the walls of the heart are thicker than they should be. Instead of benefiting from a stronger heart, this condition makes it more difficult for the cat to pump blood to the rest of the body. Kidney Disease (PKD): It is a genetic mutation that leads to the development of benign cysts in the cat’s kidneys and other organs. It is a hereditary disease that’s fairly common for Siberian cats. Gum Disease: Many Cat Owners overlook the importance of dental hygiene in their furbabies, but with this breed, regular teeth brushing is crucial. Hereditary Cancer: Cancer is by far most common in the white Siberian Forest Cats, and can be linked to a specific pedigree lineage of “Gesha Olenya Krasa” and “Dolka Olenya Krasa”. Cats of this descent are known to have cancer-causing genes , known as oncogenes. However, as in most other animals with oncogenes, the presence of the gene doesn’t necessarily guarantee the presence of cancer, and other factors may help prevent its manifestation such as a healthy diet and regular checkups. Urinary Tract Disease: Also referred to as Urinary Crystals, the condition involves the formation of stone-like minerals, crystals and organic matter and reside in the cat’s urinary tract. This covers anything from kidney stones to blockages to infections of the kidney. Although it’s not completely known whether it’s completely hereditary, it’s very common in the Siberian Cat. Sources: http://www.vetstreet.com/cats/siberian https://www.siberiancatworld.com/siberian-cats-health-problems/ http://aubreyamc.com/feline/siberian/ https: //www.madpaws .com.au / blog / siberian-cat / Photo credit: https://cattime.com/cat-breeds/siberian-cats#/slide/1 https://cats.lovetoknow.com/Siberian_Cats
Understanding FPV and its Threat to Our Cats
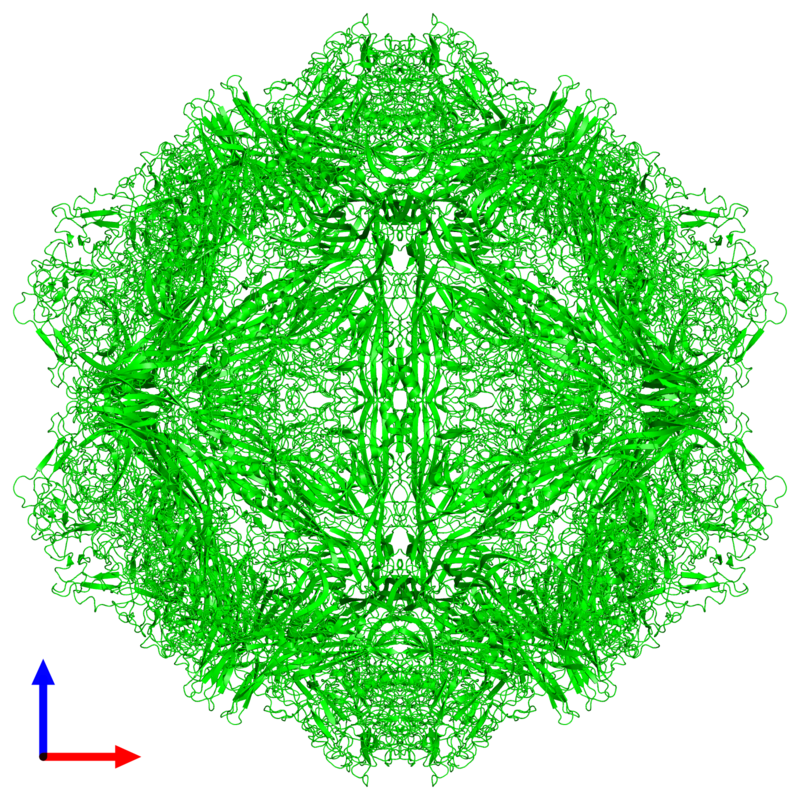
Understanding FPV and its Threat to Our Cats Maigan Espinili Maruquin The Feline Panleukopenia (FPL) is an important disease in cats. It is highly contagious and is often fatal to cats (Van Brussel, Carrai et al. 2019). This is caused by feline parvovirus (FPV; formerly FPL virus) and canine parvovirus (CPV), however, CPV infections in cats are uncommon (Barrs 2019). The FPL is also known to be the oldest known viral disease in cats wherein several epizootics that killed domestic cat populations in the 1800s could have been infected by FPV (Fairweather 1876, Barrs 2019) (Scott FW, 1987). Structure Fig. 01 A front view 60- meric assembly of FPV by Protein Data Bank in Europe containing 60 copies of Capsid protein VP1 ( https://www.ebi.ac.uk/pdbe/entry/pdb/1fpv ) The current taxonomic entity of FPV shares with CPV (Tattersall, 2006) wherein after crossing species barriers, CPVs evolved from FPV by acquiring five or six amino acid changes in the capsid protein gene (Truyen, 1999) (Appel, Scott et al. 1979, Black, Holscher et al. 1979, Osterhaus, van Steenis et al. 1980, Parrish 1990, Johnson and Spradbrow 2008, Stuetzer and Hartmann 2014, Barrs 2019). The causative agent FPV is a member of the genus Protoparvovirus in the family Parvoviridae with 5.2 kb long single stranded DNA genome, containing two open reading frames (ORFs): the first ORF encodes two non-structural proteins, NS1 and NS2; and the second ORF encodes two structural proteins, VP1 and VP2 (Reed, Jones et al. 1988, Zhou, Zhang et al. 2017). At first, FPV was thought not to infect cats (Truyen, Evermann et al. 1996). It replicates in thymus and bone marrow but not within the intestinal tract of dogs (Truyen and Parrish 1992, Truyen, Gruenberg et al. 1995). The pathway of viral entry into cells is not fully characterized, however through the feline transferrin receptor (TfR), FPV binds and uses the receptor to infect feline cells (Parker, Murphy et al. 2001, Hueffer, Govindasamy et al. 2003). However, CPV-2b and CPV-2c variants emerged, with only a single amino acid position different from CPV-2a, and infect cats both naturally and experimentally (Mochizuki, Horiuchi et al. 1996, Truyen, Evermann et al. 1996, Ikeda, Mochizuki et al. 2000, Nakamura, Sakamoto et al. 2001, Gamoh, Shimazaki et al. 2003, Decaro, Desario et al. 2011, Zhou, Zhang et al. 2017, Van Brussel, Carrai et al. 2019). FPV Infection The virus may be shed in feces even in the absence of clinical signs (subclinical infections), or before clinical signs are detected (Barrs 2019). The major portals of the FPV are the gastrointestinal (GI) tract and, less commonly, the respiratory tract. Generally, CPV is an uncommon cause of FPL and to date, no large-scale outbreaks of FPL have been confirmed to be caused by CPV (Barrs 2019). There were cases of indistinguishable CPV from FPV clinical signs in several cats (Mochizuki, Horiuchi et al. 1996, Miranda, Parrish et al. 2014, Byrne, Beatty et al. 2018, Barrs 2019). Moreover, coinfections of CPV and FPV were also reported in cats with clinical disease (Battilani, Balboni et al. 2011, Battilani, Balboni et al. 2013, Barrs 2019). The FPV can remain latent in peripheral blood mononuclear cells of healthy cats with high virus-neutralizing titers (Ikeda, Miyazawa et al. 1999, Miyazawa, Ikeda et al. 1999, Nakamura, Ikeda et al. 1999, Barrs 2019). The development of immunity of an unvaccinated cat to FPV is likely to increase with age (DiGangi, Levy et al. 2012). However, FPL mostly infects unvaccinated and incompletely vaccinated kittens. The age susceptibility correlates with the declining maternally derived antibodies (MDAs) as well as “the immunity gap” in incompletely vaccinated kittens (Barrs 2019). Clinical Signs/ Pathogenesis The FPV is resistant to heating (80C for 30 min) and low pH (3.0) (Goto, Yachida et al. 1974). Virions enter cells by endocytosis (Hueffer, Palermo et al. 2004). Viral DNA is released from the capsid and replicates through double-stranded RNA intermediates in the nucleus of the cell using the host’s DNA polymerase (Barrs 2019). It can be transmitted by the faecal-oral route and a contact with infected body fluids, faeces, or other fomites, as well as by fleas primarily spreads the virus. Viral replication primarily occurs in lymphoid tissue, bone marrow and intestinal mucosa in infected cats older than 6 weeks of age (Csiza, De Lahunta et al. 1971, Csiza, Scott et al. 1971, Parker, Murphy et al. 2001). Infection outcome ranges from subclinical to peracute infections with sudden death within 12 h (Stuetzer and Hartmann 2014). Initially, non-specific signs such as fever, depression, and anorexia during the acute stage (Addie, Jarrett et al. 1996). However, vomiting unrelated to eating occurs commonly and, less often, cats develop watery to haemorrhagic diarrhoea later in the course of disease, while some cats show extreme dehydration. Cats typically die of complications. Viral DNA can persist for long periods even after infectious virus has been lost, thus detection of DNA does not necessarily signify an active infection (Stuetzer and Hartmann 2014). Utero infection in early pregnancy can result in foetal death, resorption, abortion, and mummified fetuses while in later pregnancy may damage the neuronal tissue. The main clinical signs of FPV infection for new- born kittens include neurological, with ataxia, hypermetric movements and blindness, while some also shows signs of cerebellar dysfunction, forebrain damage (with seizures) with a range of severity and neurological signs. Although some kittens acquire MDAs, they can still get the virus for up to 2 months after birth (Csiza, Scott et al. 1971, Csiza, Scott et al. 1971, Stuetzer and Hartmann 2014). Infections occurring up to 9 days of age can also affect the cerebellum. Cats having mild cerebellar dysfunction may retain good quality of life. On the other hand, FPV can also cause retinal degeneration in infected kittens, with or without neurological signs (Percy, Scott et al. 1975, Stuetzer and Hartmann 2014). Diagnosis It is important to have the FPV detected early using accurate testing methods to prevent disease transmission
Canine HbA1c
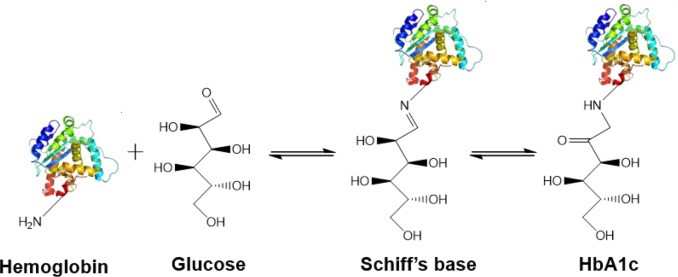
Canine HbA1c LIN, WEN-YANG (WESLEY), Ph.D HbA1c is a type of hemoglobin on which several monosaccharides such as glucose, galactose and fructose tend to bind with and exist in the bloodstream. The chemical linking process between sugar and hemoglobin is named glycation. HbA1c is actually an indicator of the beta-N-1-deoxy fructosyl on hemoglobin, which has been used as diagnostic measurement of long-term glycemic control for patients with diabetes mellitus. The increasing number of HbA1c in bloodstream represent the elevated plasma glucose level that usually indicating a poor diabetic management may lead to severer conditions. Due to the lifecycle of red blood cells is average four months, the HbA1c test could effectively present the real blood sugar degree in latest three months. In 1980, Wood and Smith first demonstrated the applicable method of examining canine diabetes with monitoring glycosylated haemoglobin. Later, Smith et al 1982, Mahaffey and Cornelius 1982, Dennis 1989, Jensen 1995 all confirmed this method is workable on diagnosing diabetic dogs. Molecular structure of HbA1c Figure 1 showed an aldimine linkage between glucose and hemoglobin (Figure1). Figure 1. Glycation process between Hemoglobin and glucose https://www.sciencedirect.com/science/article/pii/S0956566318304500 As we previously discussed that different monosaccharide would form different chemical structure with blood cells. Thus, while using cation exchange chromatography to separate Hemoglobin type A, different fractions would be separate out like HbA0, HbA1a, HbA1a2, HbA1b, and HbA1c. Fractions were named after eluting order (Figure 2). Figure 2. Different separated fractions of Hemoglobin type A with cation exchange chromatography https://www.sciencedirect.com/science/article/abs/pii/S0003269711000753 Glycated hemoglobin forming harmful factors in human body Highly reactive free radicals promote the formation of abnormal ferryl Hb (Fe4+-Hb), which enhance macrophage accumulation in blood vessel. Both macrophage accumulation and accumulation of glycated hemoglobin would elevate blood viscosity and slow down normal blood flow. Thus, atherosclerotic plaque would gradually occur in blood vessel. History of standardized HbA1c as diagnostic tool for diabetes Diabetes has become a serious health issue in entire world, due to over 220 million patients are suffering of it. Among all cases of diabetes, type 2 diabetes take majority part (90% to 95%). Besides, worsen type 2 diabetes could also cause extra complications, such as cardiovascular diseases, peripheral neuropathy, nephropathy, optic neuropathy, diabetic foot and even unto death. Several risks factor related to diabetes including high fat diet, obesity, smoking, elevated cholesterol levels, high blood pressure and lack of regular exercise. For preventing and relieving conditions of diabetes, taking healthy diet and regular exercise are important. In addition, diabetic patients’ blood sugar condition should be monitored regularly all the time. There are many clinical methods to evaluate glycemia like urine glucose, random or fasting plasma glucose etc. However, HbA1c is considered to be one of the most accurate and efficient method for measuring long-term blood sugar level (3 to 4 months). From 1894 to 1993, Diabetes Control and Complications Trial (DCCT) established the big data of diabetic patients’ HbA1c values to mean blood glucose resulted in making HbA1c a reliable index of mean blood glucose. However, DCCT haven’t standardized the HbA1c assay methods for labs and clinics. Later, the American Association for Clinical Chemistry (AACC) Standards Committee established a HbA1c Standardization Subcommittee to develop a plan for standardizing HbA1c assay that clinical laboratories could take advantage of it to perform precise glycemic evaluation and control. Now, the National Glycohemoglobin Standardization Program (NGSP) continue to develop reliable assays of HbA1c. Diagnosing canine diabetes with HbA1c 2018 American Animal Hospital Association suggested guidelines of diagnosis and assessment for animal diabetes. Clinical evaluation of animal diabetes (cats and dogs) include hyperglycemia, physical exam, complete blood count [CBC], Elevated blood glucose (BG), glucosuria, chemistry with electrolytes, urine analysis with culture, urine protein creatinine ratio (UPC), triglycerides, blood pressure (BP), and thyroxine (T4). While the level of BG concentration elevated to 200 mg/dL in dogs and 250–300 mg/dL in cats, glucosuria will typically occur. Pets with persistent glucosuria, persistent hyperglycemia, and presence clinical signs would be judged as diabetes mellitus (DM). Furthermore, HbA1c now have become a crucial glycemic indicator for diabetic dogs. Neslihan Tascene et al. revealed that blood HbA1c levels of diabetic were found to be 3.11±0.4 %, whereas the normal dog was 1.07±0.08 % respectively. Besides, the blood serum glucose level of diabetic dogs was around 526.71±22 mg/dl, whereas blood sugar in control dogs were around 97.80±2.93 mg/dl. Hasegawa S. demonstrated 6.41% HbA1c in diabetic dogs, whereas normal dogs with 2.6% (mean HbA1c of total Hb, %). And mean HbA1 values of normal dogs and diabetic dogs were 3.58 and 7.41%. In addition, Na-Yon Kim et al. showed significantly higher HbA1c concentrations of diabetic dogs (>6.2%) than non-diabetic dogs (p < 0.001) with commercial HbA1c testing system. Furthermore, Chao-Nan Lin et al. present the stability of canine glycosylated hemoglobin sample at room (25°C) and refrigerator (4°C) temperatures over 14 days. Besides, different purified methods would cause slight variation in measuring HbA1c value. Monitoring of pets’ diabetes Monitoring options include performance of blood glucose curves (BGCs), monitoring urine glucose (UG), measuring fructosamine, and assessment of clinical signs and weight. a. Blood glucose (BG) levels: Blood glucose levels fluctuate and could be used for indicating short periods of hyperglycemia. Normal BG were 63~110mg/dL in dogs; 47~151mg/dL in cats. As we mentioned, when the BG concentration goes over approximately 200 mg/dL in dogs and 250–300 mg/dL in cats, Glucosuria will occur. Blood Glucose Curves should be established during insulin treatment. b. Threshold of urine glucose (UG): UG concentration reflects only the average BG. Thus, it’s not recommended to solely rely on UG measurements is not recommended. Regardless, UG concentration can assist in assessment of DM together with other evaluation parameters. The threshold of urine glucose (UG) would fall in 180mg/dL in dogs; 252mg/dL in cats. c. Fructosamine: Fructosamine is a glycosylated protein formed by nonenzymatic, irreversible binding of glucose to serum albumin. It able to discern normal glycemic level from diabetes with chronic hyperglycemia and won’t be affected by
Case study: Feline infectious peritonitis in a cat presented because of papular skin lesions
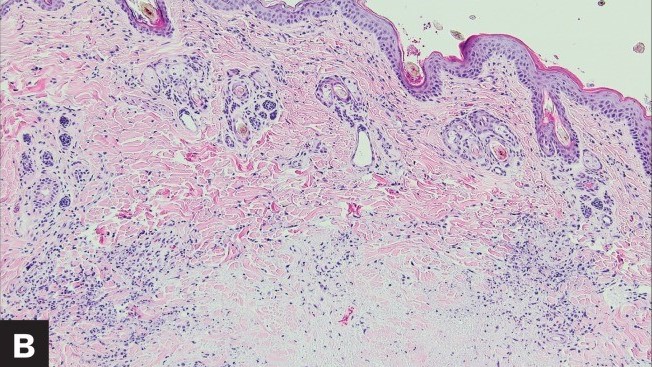
Case study: Feline infectious peritonitis in a cat presented because of papular skin lesions Robert Lo, Ph.D, D.V.M A 19-week-old neutered male domestic shorthair cat presented only multiple raised pruritic skin lesions along the dorsal head and back and no other symptoms. The cat showed poor appetite and spreading of the skin lesions five days after initial treatment, and then biopsies were taken and submitted to the dermatopathology service. Histopathology indicated strong suspicion of FIP. With cat’s health getting worse, euthanasia was performed. Then, necropsy was also performed. Abundant viscous serofibrinous effusions were found in the abdomen, thorax, and pericardium. Multiple white nodules were observed in the lungs, liver, and kidney. Histologic examination revealed multifocal to coalescing areas of pyogranulomatous inflammation in the affected tissues. Dermal necrosis was observed in skin sections. Immunohistochemical staining for intracellular feline coronavirus showed positive staining within the cytoplasm of macrophages in the lung, kidney, skin, and brain. Feline infectious peritonitis with associated cutaneous lesions was diagnosed in this cat based on gross and histologic lesions along with immunohistochemistry results. Figure 1: A — Skin lesions (multiple round raised skin nodules) on the dorsal surface of the head and neck. B —Dermal necrosis was observed in histological sections of skin; H&E. C —Immunohistochemical staining for intracellular feline coronavirus showed positive brown staining in skin section. (Redford T, Al-Dissi AN. Feline infectious peritonitis in a cat presented because of papular skin lesions., Can Vet J. 2019 Feb;60(2):183-185.) Original paper: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6340254/
