Webinar: How to Solve Annoying Canine Babesiosis in Clinics- Diagnosis and Treatment

Bioguard Corporation participate.
Sudden spike in rabies cases a cause of concern for India
In India, dogs are responsible for about 97 per cent of human rabies, followed by cats (2 per cent), jackals, mongoose and others (1 per cent). The disease is endemic throughout the country. It infects the central nervous system, ultimately affecting the brain and resulting in death. The time lag between the bite to rabies and onset of symptoms of the disease, is usually about a few days to few months in humans, depending on the site and severity of exposure. Rabies is one of the oldest recognized Zoonotic diseases with almost hundred per cent fatality and has evolved as an important public health concern worldwide. According to some studies, around 174 lakh persons are bitten annually worldwide and more than 55,000 people die annually of the disease. The sudden rise in cases of dog bits in India has become a cause of concern for all. One incident of a pet dog biting a minor boy inside the lift of a society of in Ghaziabad was a shocker and compelled everyone to think about random dog bites and steps to prevent it. According to available data, India reported total 72,77,523 cases of animal bites in 2019, which dropped to 46,33,493 in 2020 and 17,01,133 a year later. The first seven months of 2022, however, recorded more than 14.5 lakh cases. The national guidelines for rabies say that it is an acute viral disease that causes fatal encephalomyelitis in virtual all the warm-blooded animals including humans. The virus is found in wild and some domestic animals and is transmitted to other animals and to humans through their saliva. Read more.
WEBINAR: The Feline Thyroid Gland and its Hyperfunction

Bioguard Corporation participate.
Feline Herpesvirus Infection- Diagnosis

Trinh Mai Nguyen Tang Feline herpesvirus-1 (FHV-1) is a feline respiratory infection virus also known as feline viral rhinotracheitis (FVR) [1]. The Herpes virus was first isolated by scientists Crandell and Maurer in 1958 in cats with respiratory infections [2]. This virus has a prominent genome with large double stranded DNA, belonging to the family Herpesviridae [3]. This virus is characterized by cat-to-cat transmission with an exposure rate of up to 97% [4]. Herpes virus can be inactivated at 37oC around 3 hours or at 56oC in 5 mins. Meanwhile, the virus can remains infective in the enviroment approximately 5 months and a month at 25oC [5]. Once a cat is infected with the herpes virus, it is incredibly difficult to completely treat it since the virus can enter a dormant state and continue to survive in the cat for the remainder of its life [6]. Cats are not infectious during this latent period, but if they are sick or going through a stressful period, the virus may be reactivate. If this occurs, the cat will once more get the infection and may represent symptoms [7]. Herpes viruses can be latent in the ganglion, attach to sensory nerves and reach nerve cells, persist in the nucleus of infected nerve cells and do not replicate, leading to the process of detecting this virus becomes difficult [7-8]. As reported by Ngoc.N.T and her colleagues, herpes virus can infect cats of any age, however, kittens are more susceptible [9]. Specifically, the prevalence of virus infection in cats younger than 6 months old, 6-12 months old and over 12 months old were 52.17%, 33.33% and 19.05%, respectively [9]. Although previous reports have demonstrate that gender has no effect on the incidence of herpes virus infection [7-8], but Henzel et al. (2002) found that isolates from female cats are substantially taller than isolates from male cats [10]. Clinical Symptoms Herpes virus enters the cat’s body by contact with infected tears, nose, saliva, or items, and then multiplies rapidly in the epithelium of the nose, nasopharynx, and conjunctival mucosa leading to primary infection [11]. In cats infected with FHV-1, signs of sadness, moodiness, lethargy, sneezing, fever, and discharge from the eyes and nose have been noted (figure 1-A), a process that is frequently extended 2-4 days or longer, depending on the immunological system of the cat [11]. Secondary infection occurred after the fourth day of incubation, with symptoms of infection in the throat, bronchi, and bronchioles, and the nasal and conjunctival epithelium necrosing [12]. Conjunctivitis is a common herpes virus symptom, indicated by congestive and exudative symptoms that develop over many days to purulent discharge (figure 1-B) [13]. Gaskell and Dawson (1988) found lung infection or bronchitis in cats, with kittens dying from pneumonia at a greater incidence than adult cats [11,14]. Some other atypical symptoms such as mouth and skin ulcers, dermatitis or neurological signs are rarely seen [7]. Furthermore, the mean white blood cell (WBC) count of cats infected with FHV-1 (17.77 ± 0.70 x103/μl) was slightly increased compared with that of normal cats (4.6-12.8 x 103/μl) [9], in which neutrophils, eosinophils and monocytes all showed signs of slight increase compared. Secondary infections of the eyes, upper respiratory tract, and necrotic ulcers of the mouth can all cause high white blood cell counts [11]. Table 1 displays the white blood cell count. Table 1. Hematological results of cats was infected with FHV-1 [9]. Targets Unit Reference ± SE Red blood cells ´ 106/μl 7-10,7 10,20 ± 0,64 Hemoglobin content g/dl 11,3-15,5 13,47 ± 0,54 RBC mass % 33-45 38,78 ± 1,41 Average volume of red blood cells fl 41-49 45,16 ± 0,75 Average amount of hemoglobin in red blood cells pg 14-17 15,57 ± 0,24 Platelet count ´ 103/μl 180-680 362,50 ± 30,82 WBC count ´ 103/μl 4,6-12,8 17,77 ± 0,70 Lymphocytes ´ 103/μl 1,05-6,00 4,50 ± 0,36 Mono leukocytes ´ 103/μl 0,05-0,68 0,96 ± 0,13 Neutrophils polymorphonuclear leukocytes ´ 103/μl 2,32-10,01 11,47 ± 0,45 Eosinophils ´ 103/μl 0,1-0,6 0,78 ± 0,08 Basophils ´ 103/μl 0-0,14 0,07 ± 0,01 Laboratory Diagnosis In the laboratory, there are many different methods used to determine FHV. Common approaches include PCR, virus isolation in cell culture, and indirect fluorescent antibody staining of tissue samples for viral antibody detection [8,16]. PCR FHV is one of the most common causes of upper respiratory tract illness in cats. Infected cats would show upper respiratory signs. Co-infection of FHV with other pathogens makes the clinical signs more severe, particularly feline calicivirus, Chlamydophila felis, Bordetella pneumoniaseptica, Mycoplasma species, Staphylococcus spp., or Escherichia coli [6]. When a cat is suspected of having a viral infection, a swab can be used to collect nasal, ocular, oropharyngeal secretions, corneal debris, aqueous humor, corneal samples, blood, or biopsies. By amplifying viral DNA, tPCR can detect genetic material of FHV in specimen. However, if the cat is not in the infectious phase, no virus particles will be shed, rendering the PCR test ineffective [19-20]. ELISA method – detecting IgG antibodies The enzyme-linked immunosorbent test (ELISA) is used to determine IgG antibodies against FHV or FHV-1 utilizing serum, aqueous humor, and cerebrospinal fluid samples [15]. This approach, however, cannot discriminate between diseased and vaccinated cats. Due to the extended latent period of FHV, the cat’s body will generate antibodies to combat it [8]. These neutralizing antibodies manifest 20-30 days after the first infection. As a result, the presence of antibodies in the serum signals a prior infection but does not always correspond with clinical signs [8]. Immunofluorescent antibody assay Another approach for detecting FHV is immunofluorescence antibody (IFA) testing on corneal or conjunctival smears or biopsiengs. Through an antigen-antibody response, this assay may identify viral proteins produced in cells. However, this approach is thought to be less sensitive than viral isolation or PCR [16]. Virus isolation This is a traditional method that can detect viruses through isolation of conjunctival debris, nose, oropharynx, or postmortem lung samples from infected cats [8]. This traditional method can detect
Canine Lyme Disease
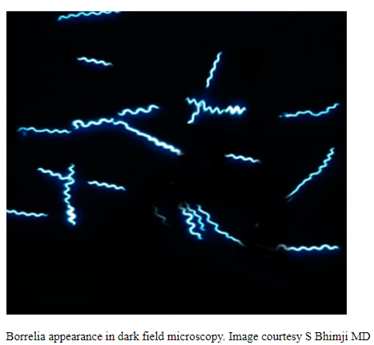
Oliver Organista, LA Lyme disease is a disease caused by the bacterium Borellia burdorgferi; a worm like, spiral-shape bacterium of spirochete class in the genus Borellia. The bacterium B. burgdorferi is transmitted through a bite of infected blacklegged tick or deer tick (Ixodes scapularis) to dogs and humans[1]. Different life-stage of I. scapularis ticks emerge at different times of the year (varies according to geographic location), giving a seasonality to Lyme disease transmission dynamics. It appears primarily in specific areas including the southern New England states; eastern Mid-Atlantic states; the upper Midwest, particularly Wisconsin and Minnesota; and on the West Coast, particularly northern California in the United States. It is also present in Europe and Asia[7]. Most of the areas where to find them are in forest or grassy, wooded, marshy areas near rivers, lakes or ocean, and are common in homes and buildings in secluded or rural areas. In Canada, there 2 types of blacklegged or deer tick that can spread Lyme disease. The blacklegged tick (Ixodes scapularis) and the blacklegged (Ixodes pacificus) [3] . Dogs tend to be bitten by infected I. scapularis adults, which are most active in the cooler early spring and late fall months [2]. An adult female tick is rarely (if ever) transmitted the B. burgdorferi to her offspring. Ticks most commonly become infected as juveniles after a bloodmeal on an infected wildlife host (most commonly rodents). Because ticks typically feed only one time per life stage, the next opportunity for B. burgdorferi transmission is during the next bloodmeal in the tick’s next life stage[2]. Typically, Lyme disease symptoms will take a couple of months or more to appear (2-5 months) after getting infected [8]. Symptomatically, Lyme disease can be difficult to distinguish from anaplasmosis because the signs of the diseases are very similar, and they occur in essentially the same areas of the country. Lyme disease is diagnosed through a blood test that shows whether an animal has been exposed to the bacterium[11]. Common symptoms that will appear are: Lameness: An inability to use one or more limbs is one of the most common symptoms of Lyme disease in dogs. Swollen lymph nodes: found in the neck, chest, armpits, groin, and behind the knees, are typically the first to show swelling. Lymph node swelling indicates an immune response triggered to fight the disease. Joint swelling: Swollen joints, stiff walking, or avoidance to touch may be other signs of the disease. Fatigue: Dogs with Lyme disease may also exhibit flu-like symptoms of low energy and lethargy. Loss of appetite: Losing interest in eating, especially if it leads to weight loss, is another sign that a dog may have Lyme. Fever: In addition to the above symptoms, a dog may have a fever caused by the Lyme disease infection. In rare cases, if Lyme disease is left untreated it can lead to damage in the kidneys, nervous system, and heart. Lyme disease affecting the kidneys is the second most common syndrome in dogs and is generally fatal. Facial paralysis and seizure disorders have been reported in the disease form affecting the nervous system. The form of the disease that affects the heart is rare. [10]. The most commonly used to diagnose Lyme disease in dogs are the serologic assays. Although some laboratories still use traditional serologic methods (e.g., whole-cell enzyme-linked immunosorbent assay and immunofluorescence assay), these assays have largely been replaced by serologic assays that detect host antibodies to specific B. burgdorferi proteins. These assays are qualitative, providing a yes/no answer regarding B. burgdorferi serostatus[3]. Treatment is generally recommended for seropositive dogs that display clinical signs of Lyme disease or are asymptomatic but have evidence of protein-losing nephropathy[4]. Most frequently antibiotics used to treat Lyme disease in dogs are doxyclycline and monicycline, at a dosage of 10mg/kg PO q12h to q24h for 30 days [2][5]. Amoxicillin and erythromycin are other antibiotics that can be used for treating the disease. A non-steroidal anti-inflammatory (carprofen or deracoxib) may also be given to the patient [6]. A possible complications may occur when treating Lyme disease. Some dogs who take antibiotics can develop loss of appetite, vomiting and diarrhea. Once infected, a dog will always have the bacteria that cause Lyme disease in his or her body. Therefore, relapses are possible; lookout for unexplained fever, swollen lymph nodes, and/or lameness. A small percentage of dogs develop kidney failure as a result of Lyme disease. Clinical signs include vomiting, weight loss, poor appetite, lethargy, increased thirst and urination, and abnormal accumulations of fluid within the body. [6] The best way to protect from Lyme disease is to use tick-preventive products year-round. Several safe and effective commercial parasiticides are available for tick control on dogs and cats, including systemics (isoxazolines), topicals (permethrin, fipronil), and collars. Another effective strategy is vaccination. Other prevention strategies include reducing exposure to ticks and avoiding areas with ticks [2]. References [1] Lyme Disease Diagnostic Market – Growth, Trends, COVID-19 Impact, and Forecasts (2022 – 2027), MOdor Intelligence, January 202 [2] Lyme Disease in Dogs: Signs and Prevention, Kathryn E. Reif, MSPH, PhD.,April 2020, https://todaysveterinarypractice.com/parasitology/lyme-disease/ [3] Lyme Disease, IPAC (https://ipac-canada.org/lyme-disease.php) [4] / Littman MP, Gerber B, Goldstein RE, et al. ACVIM consensus update on Lyme borreliosis in dogs and cats. J Vet Intern Med 2018;32(3):887-903. [5] Mullegger RR. Dermatological manifestations of Lyme borreliosis. Eur J Dermatol. 2004 Sep-Oct;14(5):296-309. PMID: 15358567 [6] How to Treat Lyme Disease in Dogs, Jennifer Coates, DVM, December 2014, PETMD (https://www.petmd.com) [7] Lebech AM. Polymerase chain reaction in diagnosis of Borrelia burgdorferi infections and studies on taxonomic classification. APMIS Suppl. 2002;(105):1-40. PMID: 11985118 [8] Could Your Dog Have Lyme Disease? How to Recognize the Symptoms and Get Treatment, Lavanya Sunkara , July 2022, GoodRx Health [9] Everything You Need To Know About Lyme Disease In Dogs, Kimberly Alt, July 2022, Canine Journal [10] Lyme Disease (Lyme Borreliosis) in Dogs, Reinhard K. Straubinger, DrMedVetHabil, PhD, Institute for Infectious Diseases and Zoonoses, Department of Veterinary Sciences, Faculty of Veterinary Medicine, LMU, October 2022 [11] Lyme disease: A pet owner’s guide, American Veterinary Medical Association
Psittacine Beak and Feather Disease

Long Pham Introduction Psittacine beak and feather disease (PBFD) is an infectious viral disease that infects psittacine birds. This disease affects Old World (Australian and African) psittacine birds and New World (Americas) psittacine birds (Greenacre, 2005). The peracute and acute form of this disease can cause sudden death, while the chronic form of this disease damages the feather, deforms the beak, and will eventually lead to death. (KATOH et al., 2010) The disease is caused by a small circovirus, which is a single-stranded DNA virus belonging to the Circoviridae family (Hakimuddin et al., 2016). The virus can spreads through direct contact with contaminated surfaces, feces, feather dander, and other bodily excretions (Greenacre, 2005). It can be transmitted horizontally to other birds in the same generation and vertically to eggs and young chicks in the next generation (Hakimuddin et al., 2016). Since the virus has a non-envelope structure, it is able to resist many control measures and is able to persist in the environment and infected substances for a long time. The origin of PBFD was thought of to be from Australia (PASS & PERRY, 1984), where it then spread to the rest of the world. Possibly through pet trades and import of these birds, this disease was able to spread globally. Report of this disease has occurred in other countries located in North America, Europe, Africa, Asia, and even on islands in the Indian and Pacific Oceans (Harkins et al., 2014). Psittacine beak and feather disease prevalence around the world varies and has been reported to be around 41.2% in Taiwan (Hsu et al., 2006), 3.5–4% in USA (de Kloet & de Kloet, 2004) and 23% in Australia (Khalesi et al., 2005). With an increasing trend of live birds being traded globally, the spread of PBFD and other diseases will surely grow. Diagnosis Typical clinical signs of PBFD include lethargy, weight loss, shedding and abnormal development of feathers, beak elongation and deformation, and eventually death (PASS & PERRY, 1984). This disease can occur in three different forms: peracute, acute, and chronic. Progression of the disease depends on the age, with younger birds having a higher progression rate (Greenacre, 2005). Some symptoms of peracute PBFD are weight loss, pneumonia, sepsis, enteritis, liver necrosis, and leukopenia (Schoemaker et al., 2000). Sudden death is likely to occur in peracute PBFD. In acute PBFD, majority of those affected by this phase are between the ages of 0-3 years old and it is thought that their susceptibility is based on their condition instead of the virus’ antigenic or genotypic characteristics (Ritchie et al., 1990). Some clinical signs includes depression and rapidly developing feather dystrophy can occur, affecting 80-100% of the feathers in as little as one week (Ritchie, 1995). Sudden death can also occur in this form. Those that survive this phase will have an incubation period, which may be years, before going to the chronic PBFD phase (Greenacre, 2005). For chronic PBFD, it is typically characterized by symmetrical feather dystrophy that progresses slowly and gets worse over time (Greenacre, 2005). Birds can become completely bald and can have beak deformities (Figure 1), where the beak becomes elongated. Death usually occurs from secondary infections, fungal or bacterial, because lymphoid tissues are usually damaged by the virus and causes the immune system to be suppressed (Ritchie et al., 2003). Figure 1: Cockatoo with advanced PBFD (Harcourt-Brown, 2009) PBFD can be diagnosed successfully from just careful examination. The disease can first be suspected if the bird is progressively losing feathers or has a symmetrical feather dysplasia. However, a loss of feathers does not always mean it is PBFD as the cause can be from other reasons, such as being self-inflicted or from excessive allopreening, which causes injuries that look similar to those caused by the disease (Wellehan et al., 2016). PBFD can be diagnosed though antigen and antibody detection from hemagglutination assay and hemagglutination inhibition. In addition, polymerase chain reaction (PCR) is also used for detecting PBFD, being a standard method of detection in most countries (Wellehan et al., 2016). False positives can occur with this method due to the nature of the virus to easily contaminate and persist in the environment, which will contaminate the samples, such as feathers, that are exposed to this environment (Wellehan et al., 2016). Therefore, the choice of sample collection method can have a major impact to the results. In one study, it was found that the use of blood samples for a PCR test resulted in 47 out 56 birds being positive for PBFD, while only 10 birds had a positive result when feather samples were used (Khalesi et al., 2005). Treatment and Disease Control Current treatment for PBFD is for supportive care to prevent secondary infections as there is no cure for this disease. The disease is fatal when clinical signs appear, while other birds that has an immune response and don’t show any clinical signs, making this naturally vaccinated (Greenacre, 2005). Effective methods of controlling this disease involves isolating suspected carriers, testing, and if necessary, culling to prevent a possible outbreak from occurring. The resilience of the virus to many chemical disinfectants and even extreme temperatures can be based on the physicochemical properties of the virus (Raidal & M.Cross, 1994). However, Virkson S or other peroxide disinfectants have been suggested for use to disinfect contaminated areas (Wellehan et al., 2016). Strict hygiene practices with the right disinfectants is the key to prevent further spread of PBFD. While there are no vaccine for PBFD available commercially, research into developing one is ongoing and currently made vaccines appears to be effective. Future control of the disease will still depend on implementing strict hygiene practices and testing methods since vaccinated birds may still spread the disease. References Greenacre, C.B. (2005) Viral diseases of companion birds. Veterinary Clinics of North America: Exotic Animal Practice, 8, 85–105. KATOH, H., OGAWA, H., OHYA, K. & FUKUSHI, H. (2010) A review of DNA viral infections in Psittacine birds. Journal of
Webinar: “Canine Hypothyroidism: Diagnosis & Treatment”

Webinar: “Canine Hypothyroidism: Diagnosis & Treatment”

Webinar: “Canine Hypothyroidism: Diagnosis & Treatment”

Webinar: “Canine Hypothyroidism: Diagnosis & Treatment”

