Feline Polycystic Kidney Disease (PKD)
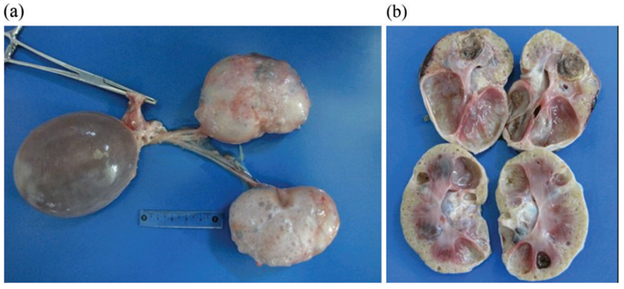
Maigan Espinili Maruquin Introduction Cats, as a fur family, require health attention. However, some felines can get infected with certain types of inherited diseases. One of the most prevalent genetic diseases is the Feline Polycystic Kidney Disease (PKD) [1, 2], which causes the progressive development of multiple fluid-filled cysts in the kidney and in some cases extends to the liver and pancreas [2-5]. Prevalence of the PKD in Persian cats has been studied in different countries including, the United Kingdom (49.2%) [6], Japan (46%) [7], Australia (45%) [8], and France (40.45%) [9]. Reports suggest that the disease is not related to the sex of the feline, after results show no statistical differences between males and females [9, 10]. Different species of felines can get the said disease like Charteux [11], Neva Masquerade cat [12], and Scottish Fold and American Shorthair [7], however, it is most commonly inherited by the Persian and Persian- related breeds [3] with 35 to 57% detection via ultrasonography or genetic testing [13]. It is suspected that all the feline breeds could have inherited the mutation where the disease starts, considering that around 80% of all current feline breeds have had some type of cross with Persian breed [3, 14]. In humans, 1 in 500–1000 of the general population can inherit Autosomal dominant polycystic kidney disease (ADPKD) [15], which is characterized by increase in kidney size caused by the development and progressive enlargement of renal cysts, eventually leading to a steady decline in glomerular filtration rate [16]. The PKD1 and PKD2 genes, which are responsible for coding the polycystin-1 and 2 proteins, where 85 % and 15%, respectively, are responsible for the mutations [17]. Relative to the cases recorded with ADPKD in cats, the feline ortholog of the human PKD1 gene, which is also named PKD1, were recorded to have a single germline mutation. During the mutation, at exon 29 of the feline PKD1 gene, position 3284, a pyrimidine base (cytosine, C) is substituted by a purine base (adenine, A), wherein the premature stop codon produced causes the 25% loss in the C- Terminal formation of polycystin-1 protein [3, 18, 19]. With similar features clinically and morphologically in both human and feline ADPKD [2], feline PKD is a good representation for human ADPKD [7]. Clinical Presentations Fig. 1. Postmortem images, including cross-section of the polycystic kidneys of a seven-year-old, entire male British shorthair cat (cat 4), tested positive for the PKD1 mutation, demonstrating multiple cysts of variable sizes in both kidneys (a, b). [4] The polycystin-1 is suggested to play a role in cell–cell and matrix–cell interactions [20]. The primary cilium, where this protein is expressed, functions in fluid transport and chemo and mechanoreceptors [21, 22]. Cysts of different sizes were presented in renal cortex and medulla, sometimes occurs in the liver and pancreas, as well [8], and increases both in size and quantity as they age [23]. With slow growth and progression, affected cats start to deteriorate renal functions [7]. The cysts develop by different scenarios including the increase of cell proliferation, fluid secretion, and extracellular matrix alterations, cilia with lost polarization would then alter the water reabsorption function [3, 24]. Secondary to renal cysts development, obstruction due to nephrolithiasis, lymphoma and chronic kidney disease with interstitial nephritis are also displayed, especially in old cats [8, 25] In a study conducted among cats in Turkey, some diagnosed cats were reported for fatigue, anorexia, and vomiting. During palpation, an increase in total kidney volume was discovered, and cystic lesions were seen in the cortex of both kidneys when ultrasonography was performed [19]. Generally, apathy, anorexia, weight loss, bad appearance of the coat, polyuria and polydipsia, and gastrointestinal disorders could be observed [26-28], while general dehydration and pale mucous membranes are also noticeable on clinical examinations [3]. Elevated serum Cre concentration has been presented as one of the clinical signs in cats from three years old, while other cats at nine showed normal concentration. This suggests variable clinical courses of the disease [7]. Whereas, few cases showed hepatic cysts an extrarenal manifestation [6]. Diagnosis Imaging Diagnosis Fig. 2. Ultrasound picture of a 2.5 year old Persian cat, positive for PKD. Note the distal enhancement beyond the anechoic cyst structures (arrow). The largest cyst measures 29 mm in diameter [8]. The Ultrasound is known to be with the highest successful diagnosis, allowing quick and reliable diagnosis [23], and is considered due to availability and non-invasive, safe, cheap and effectivity in detecting the presence of kidney cysts [28]. Whereas, radiography and intravenous urography are usually used in more advanced cases, like when there is a presence of multiple, large cysts [3]. Molecular Approach Various PCR methods have been used to identify and amplify the DNA fragment of interest [3]. The RFLP-PCR was developed and used [18], real-time PCR or quantitative PCR is known to be reliable and faster than the earlier technique [29]. Whereas, ARMS-PCR (Amplification-refractory mutation system-Polymerase Chain Reaction) presented its advantages in time, low quantity of samples needed and its low cost, which has resulted in 100% sensitivity and specificity [30]. The synergistic use of genetic testing to confirm the presence of the causal mutation and make an early diagnosis; and the ultrasound to diagnose polycystic kidney disease and to monitor the progression of the disease has been agreed by several authors to plan detection programs for feline PKD [3, 30-32]. For as early as three years old, affected felines can develop signs of impaired renal function, and thus, positive cats are strongly discouraged for breeding [7]. References Young, A.E., et al., Feline polycystic kidney disease is linked to the PKD1 region. Mamm Genome, 2005. 16(1): p. 59-65. Eaton, K.A., et al., Autosomal dominant polycystic kidney disease in Persian and Persian-cross cats. Vet Pathol, 1997. 34(2): p. 117-26. Schirrer, L., P.J. Marín-García, and L. Llobat, Feline Polycystic Kidney Disease: An Update. 2021. 8(11): p. 269. Nivy, R., et al., Polycystic kidney
Canine Hypothyroidism
Sushant Sadotra Introduction: Hypothyroidism is a prevalent thyroid disorder that is caused due to the deficiency of thyroid hormone. In this condition, there is an irregular short production and improper secretion of thyroid hormones into the blood from the thyroid gland. This leads to a slow metabolic rate and loss of proper body functions. Regarding pet animals, hypothyroidism is mostly occurring among dogs and rarely in cats and other pet animals. Hypothyroidism Etiology in canines: Imbalance at any level of the hypothalamic-pituitary-thyroid axis can cause hypothyroidism in animals, especially dogs. In Adult dogs, the onset of primary hypothyroidism can cause either because of lymphocytic thyroiditis or idiopathic atrophy of the thyroid gland. The gradual destruction of follicles and secondary fibrosis on the gland because of diffuse infiltration by lymphocytes, plasma cells, and macrophages into the thyroid gland. This condition is known as lymphocytic thyroiditis. Loss of thyroid parenchyma leads to the idiopathic atrophy of the thyroid gland, in which fatty tissue will replace the lost thyroid parenchyma. The condition can occur due to autoimmune thyroiditis. Secondary hypothyroidism can be caused due to the damage of pituitary thyrotrophs by tumor growth, leading to the deficiency of one or more pituitary hormones. Hypothyroidism in dogs can occur due to other rare causes such as congenital hypothyroidism or neoplastic destruction of thyroid tissue. Congenital hypothyroidism can be primary or secondary. Thyroid dysgenesis and dyshormonogenesis can cause congenital primary hypothyroidism in dogs. Congenital secondary hypothyroidism can show clinical signs caused by the deficiency of growth hormones such as dwarfism, lethargy, gait abnormalities, or pituitary dwarfism. Diagnosis of hypothyroidism in canines: A variety of nonthyroidal factors and other conditions can mimic thyroid disorder and mislead the correct diagnosis of canine hypothyroidism. The severity and chronicity of the clinical findings associated with hypothyroidism and other clinicopathologic abnormalities of the hypothyroid state can be a basis for the choice of a proper diagnostic test. Tests that could confirm the diagnosis of hypothyroidism in canines are mentioned below: Total T4: The initial screening test for hypothyroidism can determine Total T4 concentration. A dog with a T4 concentration lower than the reference range limits may be anticipated to have a hypothyroid issue. However, it can also indicate that the dog can have a nonthyroidal illness such as sick euthyroid syndrome. Therefore, the total T4 test alone cannot be a proper diagnosis. Free T4: In serum, unbound T4 is supposed to be biologically active. Therefore, it is a very used test to differentiate between hypothyroid and euthyroid dogs. Also, a free T4 assay can give a better diagnosis with high sensitivity and specificity. Most commercial Free T4 tests use the method of single-stage solid phase (analogue) assays. However, an equilibrium dialysis step can improve the accuracy. Free T3: Among T4 and T3, T3 is the most potent hormone in animals. Therefore, measuring Free T3 is also a sensitive diagnostic step. However, during the onset of the primary hypothyroidism, serum T3 determination is weak. Also, it is found that in hypothyroidism dogs, serum T3 concentrations could be low, normal, or high. If the serum concentration of T3 is high, it is to be checked whether or not anti-T3 antibodies are producing false results in the T3 radioimmunoassay. Serum TSH concertation: In primary hypothyroidism, high serum TSH concentrations are expected that further may lead to low serum concentrations of T4 and free T4. A species-specific TSH assay can be used to check the high level of serum TSH. However, a false-negative result showing normal TSH concentrations may indicate a condition of primary hypothyroidism; a false-positive result showing high serum TSH concentrations may indicate a nonthyroidal illness in a euthyroid dog. A normal serum TSH concentration can indicate secondary hypothyroidism in a few cases. Therefore, serum TSH concentration results should always be coupled with other tests for definite confirmations. TSH stimulation test: In this particular test, bovine TSH is introduced exogenously into the dog’s body, and the thyroid gland’s response is evaluated. Firstly, a basal T4 is measured. Then bovine TSH is administrated at a dosage of 0.1 U/kg. After 6 hours, T4 levels are calculated for the second time to check the response of the thyroid gland. Result interpretations could be a no response for hypothyroidism, a normal and blunted response for the sick euthyroid syndrome. Although the TSH stimulation test is one of the accurate tests to check thyroid function, it is expensive and less available. Imaging: Thyroid Ultrasonography can detect the decreased echogenicity followed by decreased thyroid volume. The procedure can take the best imaging of the thyroid gland by technetium 99m (99mTc). This diagnostic tool can differentiate between hypothyroidism and euthyroid sickness. Therapeutic trial: In this approach, a thyroxine supplementation is given to a dog at a particular dosage. If the response is positive, the supplementation is stopped to check for the return of clinical signs related to hypothyroidism. This can confirm that the dog has thyroid-responsive diseases rather than other nonthyroidal issues. However, before starting a therapeutic trial, every attempt should eliminate nonthyroidal sickness. Also, therapeutic monitoring should be performed in case the therapy is unsuccessful. Treatment of hypothyroidism in canines: The typical treatment of hypothyroidism in dogs is the oral medication of levothyroxine (L-T4). L-T4 is a synthetic thyroid hormone that can restore blood thyroid hormone concentrations and reverse hypothyroidism’s effects. The replacement of natural with synthetic hormones will be used for the rest of the animal’s life. However, a great precaution is needed with the initial dose and tailoring of the drug. The dosages of L-T4 in d are 0.01–0.02 mg/lb (0.02–0.04 mg/kg). The drug is given every day once or twice without food. Reference: Hypothyroidism is the Most Common Hormone Imbalance of Dogs; Wendy Brooks, DVM, DABVP. Date Published: 02/26/2002, Date Reviewed/Revised: 08/16/2019. Strey S, Mischke R, Rieder J. Hypothyreose beim Hund: eine Übersicht [Hypothyroidism in dogs: an overview]. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2021 Jun; 49(3):195-205. German. doi: 10.1055/a-1367-3387. Epub 2021 Jun 22. PMID: 34157761 Mark E. Peterson. Hypothyroidism in Animals. Last
