Sushant Sadotra
Introduction:
The thyroid is one of the endocrine glands in vertebrates. The thyroid gland has a bilobed structure located below the larynx and overlays the trachea in animals. In different animals, Anatomical variations of the thyroid are primarily seen in the isthmus connecting the gland’s two lobes. The size of the gland approximates 0.20% of body weight. However, the size may increase due to iodine deficiency, ingestion of goitrogenic toxins, tumors, and hyperactivity of the gland, or maybe reduced to fibrotic due to hyperthyroidism.
Thyroid follicles are the thyroid gland’s functional units with a spherical structure composed of an inner core of the thyroglobulin-hormone complex, colloid. The colloid is surrounded by an outer monolayer of follicular cells and acts as the storage tank of thyroid hormone. The overall size of the follicles and the shape of their follicular cells may differ due to the functional activity of the thyroid gland. The dormant follicular cells are squamous-shaped compared to the tall columnar, highly active follicular cells. Other than colloid, the thyroid C-cells are interspersed between the follicles. The thyroid C-cells are the source of the hypocalcemic hormone calcitonin that is associated with calcium metabolism. The third type of tissue embedded in the thyroid gland is the parathyroid. The parathyroid is the source of the hypercalcemic hormone parathormone.
Functions of the thyroid gland:
The thyroid gland functions the same in all animals. There are four primary functions of the thyroid gland in animals; trapping the iodide, synthesis of thyroid hormones, storage, and release of hormones. All these activities of the thyroid gland are usually regulated by thyroid-stimulating hormone (TSH), a pituitary hormone. Hormonogenesis and release of thyroid hormone mainly have four stages:
Trapping of Iodide:
The follicular cells trap the circulating I– from the blood against the concentration gradient mediated by a sodium iodide symporter (NIS) protein present on the thyroid follicular cell membrane. A trapping enzyme catalyzes the trapping process via a mode of active transport catalyzed by an ATP-dependent Na+K+-ATPase. This trapping system’s high efficiency can concentrate most blood iodine in the thyroid gland. This process is stimulated by TSH and blocked by large amounts of I– or goitrogenic agents. (Figure 1)
Synthesis of Thyroid Hormones:
The trapping of I- is followed by its oxidation, catalyzed by peroxidase to form a highly active free radical I*. This reaction is also stimulated by TSH and inhibited by thyrotoxic agents (thiouracils or thioureas). At the follicular cell membrane-colloid interface, highly active I* binds to thyroglobulin, a thyroidal glycoprotein of a high molecular weight of 660 kDa. I* binds to thyroglobulin at its tyrosine moieties to form monoiodotyrosine (MIT) or a diiodotyrosine (DIT). After that, the iodinated phenyl groups of the tyrosine undergo oxidative condensation resulting in the synthesis of thyroid hormones. The thyroid gland produces two active hormones: 3,5,3’-triiodothyronine (T3) and 3,5,3′,5,-tetraiodothyronine (T4). T3 is formed by the oxidative condensation of an iodinated phenyl group of one DIT to an MIT group or of one DIT to another DIT to create T4. The inner deiodination product of the T4 is the inactive hormone is 3,3′,5’-triiodothyronine (rT3).
Storage of hormones:
Thyroid follicular cells synthesized thyroglobulin and localized it to the cell membrane for the iodination process. Iodinated thyroglobulin, also known as a colloid, is released and stored in the lumen of the follicle.
Release of hormone:
TSH stimulates the release process of hormones. TSH acts at the follicular cell membrane, the second site of action for TSH. Colloid from the lumen is taken up to the follicular membrane, where they are taken in as vesicles into the follicular cells by the process of endocytosis. Lysosomes merge with these vesicles to release lysosomal proteases that hydrolyze the colloid. Hydrolyses of colloid release their T3, T4, DIT, and MIT. Microsomal tyrosine deiodinases enzymatically degrade the released DIT and MIT, and their iodine is recycled within the follicular cell. A simple diffusion process releases the T4 and T3 into circulation. Out of the total hormone released from the gland, 90% is T4, and only 10% is T3. The phenyl group of T4 may also undergo some deiodination within the gland or in the peripheral tissues to form rT3. rT3 is an inactive form of the T3 hormone. Therefore it undergoes a degradation pathway. (Figure 1)
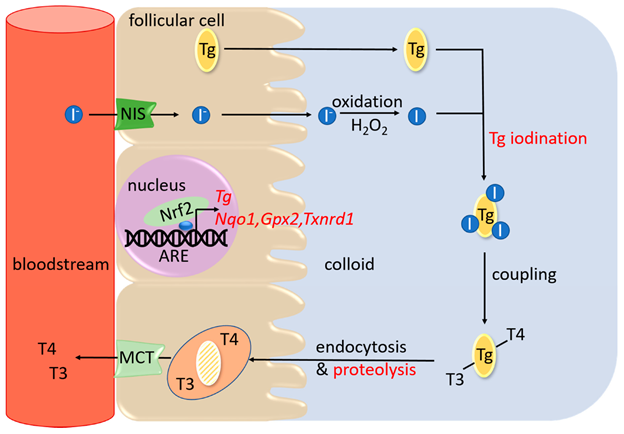
PC: Thanas C, Ziros PG, Chartoumpekis DV, Renaud CO, Sykiotis GP. The Keap1/Nrf2 Signaling Pathway in the Thyroid—2020 Update. Antioxidants. 2020; 9(11):1082. https://doi.org/10.3390/antiox9111082
The regulation of T3 and T4 secretion starts in the hypothalamus. The thyrotropin-releasing hormone secreted from the hypothalamus acts on the pituitary gland. This stimulates TSH secretion, which ultimately acts on the thyroid gland, producing and releasing thyroid hormones.
The action and disorder of Thyroid Hormones:
In humans and animals, thyroid hormones play a vital role in regulating metabolic and cellular mechanisms. The mode of action can be quick in minutes or prolonged to hours or longer. Thyroid hormones in normal levels work together with other hormones like insulin (beta cells of the pancreatic islets) and growth hormone (pituitary gland) to work on protein synthesis in different cellular processes. However, thyroid hormones can be catabolic in excess (hyperthyroidism), with increased protein breakdown and gluconeogenesis. Hyperthyroidism is more common in cats middle-aged to old cats than in dogs. However, thyroid carcinoma could be a cause when it occurs in dogs. Decreased levels of thyroid hormones (hypothyroidism) cause a slower metabolic rate in animals. This disorder is most likely seen in middle-aged (4-10 Years) dogs and mid to large-size dog breeds (Doberman pinscher, Golden retriever, etc.). Also, spayed female dogs have a higher hypothyroidism risk than unspayed ones. On the contrary, naturally occurring hypothyroidism is rare in cats.
Reference:
- Kaneko, J. J. (2008). Clinical biochemistry of domestic animals. San Diego: Academic Press.
- Thanas C, Ziros PG, Chartoumpekis DV, Renaud CO, Sykiotis GP. The Keap1/Nrf2 Signaling Pathway in the Thyroid—2020 Update. Antioxidants. 2020; 9(11):1082. https://doi.org/10.3390/antiox9111082
- Mark E. Peterson. The Thyroid Gland in Animals. Last full review/revision Jul 2019 | Content last modified Oct 2020. MSD MANUAL Veterinary Manual, https://www.msdvetmanual.com/endocrine-system/the-thyroid-gland/the-thyroid-gland-in-animals

