Andy Hua
Introduction
Classification
Taxonomy
Classically, the genus Leptospira was divided into 2 species based on genetic analysis: L. interrogans sensu latu (pathogenic strains) and L. biflexa sensu latu (saprophytic strains). L. interrogans is divided into more than 250 serovars based on antigenic composition and further classified into antigenically related serogroups. There is a serovar spectrum and frequency that differs according to countries and regions (depending on distribution of rodent hosts, import of dogs from abroad, use of vaccination). The main infecting serovars in dogs were Icterohaemorrhagiae and Canicola in Europe and America prior to 1960. Since the use of the bivalent vaccine against Canicola and icterohaemorrhagiae , OTHER serovars A to the Shift occurred .Besides. L. icterohaemorrhagiae and L. canicola , serovars Importance of Dogs in the include: grippotyphosa , Bratislava , Saxkoebing , Sejroe , Copenhagi , Australis , Bataviae , and Pomona , autumnalis , and hardjo . Icterohaemorrhagiae and Canicola infections in unvaccinated dogs still occur, indicating that these serovars are not fully eradicated. Leptospires are motile, obligate aerobe, gram-negative bacteria, which are not visible in routinely fixed smears. Dark field microscopy (Fig. 1) or phase contrast microscopy is necessary for visibility of unstained leptospires.
Clinical Effects
Epidemiology
Habitat
- Leptospires have been isolated from birds, reptiles, amphibians and invertebrates.
- Rodents and wild carnivores are the most frequent carriers.
- Reservoir hosts show few or no signs of disease.
- Leptospira spp. are commonly sequestered in the renal tubules of mammalian kidneys.
- Different serovars typically have different reservoir hosts.
Lifecycle
- Generation time in culture media or host is long.
Transmission
- Direct or indirect transmissions are possible.
- Indirect transmission through contaminated water or soil is more common.
Pathological effects
- Infection occurs through ingestion of infected rodents or penetration of mucosae or traumatized skin. Leptospiremia occurs within 1 week. Leptospires spread to other organ systems (kidneys, liver, spleen, endothelial cells, lungs, uvea/retina, skeletal and heart muscles, pancreas, and genital tract) and cause tissue damage, visceral and vascular inflammation.
- Leptospiral pulmonary hemorrhage syndrome (LPHS) Lung: pulmonary hemorrhage can occur as severe manifestation of acute leptospirosis.
- Leptospires can persist in immune privileged site (eg, renal tubes, eye).
- In the presence of adequate antibody titers, leptospires are eliminated from most organs. In the presence of low antibody titers mild leptospiremia can continue with a subclinical course of disease.
Other Host Effects
- Individual host may show little or no clinical signs but may be source of infection in the same animal species.
- An animal that has recovered may become a long-term shedder of the organism.
- Mainly dogs show disease, rodents often the reservoir.
- Cat disease is uncommon, but serology shows that asymptomatic infection occurs.
- Individual host can show little or no clinical signs but can be source of infection to other animals or humans.
- An animal that has recovered can become a long-term shedder of the organism.
- Rodents are often the reservoir.
- Dogs commonly succumb to disease if infected.
- In cats, disease is uncommon but asymptomatic infection and shedding in urine occurs.
Control
Control via animal
Antimicrobial therapy
- Dogs with gastrointestinal signs should initially be treated with intravenous penicillin derivates (eg, ampicillin or amoxicillin 20-30 mg/kg q6-8h). These should be continued until gastrointestinal signs are under control and liver enzymes are normalized. A directly following antimicrobial therapy with 3 weeks of oral doxycycline (5 mg/kg q12h) is necessary for prevention of carrier states.
- Dogs without gastrointestinal signs should immediately be treated with doxycycline.
- Antibody testing of dogs living in the same household as infected dogs is recommended. Oral doxycycline (5 mg/kg q12h for 3 weeks) should be administered, if these dogs have antibodies.
Symptomatic treatment
- Treatment of dogs with gastrointestinal sings includes antiemetics, gastroprotectants, and nutritional support.
- Use of opioids in dogs with pain can be necessary.
- Treatment of dogs with acute kidney injury (AKI) includes correction of loss of fluid, electrolytes, acid-base imbalances and hypertension, and if necessary hemodialysis for patients with persistent oligoanuria, life-threatening hyperkalemia, or severe volume overload.
- Oxygen therapy or mechanical ventilation can be necessary in dogs with LPHS.
- Plasma transfusions can be necessary for patients with DIC (disseminated intravascular coagulation).
- Whole blood transfusion can be helpful, if bleeding occurs.
Hemodialysis
- Hemodialysis is necessary in dogs with acute renal failure (life-threatening hyperkalemia or severe volume overload) and in dogs with advanced uremia refractory to medical management.
- Early referral to facilities where hemodialysis is available is recommended.
- Renal recovery usually occurs after 2-7 days of dialytic support.
- Hemodialysis leads to favourable prognosis for renal recovery (in more than 80% of dogs).
Mechanical ventilation
- Anesthesia ventilators: overview can be necessary in dogs with severe pulmonary hemorrhage due to LPHS.
Vaccination
- Vaccination protects against clinical disease and carrier status with shedding.
- Protection is serogroup-specific and temporary.
- Annual boosters are required.
Diagnosis
Leptospirosis at its onset is often misdiagnosed as aseptic meningitis, influenza, hepatic disease or fever (pyrexia) of unknown origin. Despite being common, the diagnosis of leptospirosis is often not made unless a patient presents with textbook manifestations of the so called Weil’s disease, such as fever plus jaundice, renal failure and pulmonary haemorrhage. Leptospiral infection often has minimal or no clinical manifestations; of the cases in which fever develops, as many as 90% are undifferentiated febrile illnesses. Moreover, clinicians may fail to recognize that transmission of leptospirosis can occur in the urban setting because it is incorrectly perceived to be a rural disease. Therefore, diagnosis is based on laboratory tests rather than on clinical symptoms alone. In developing countries,Laboratory facilities may be inadequate for diagnosis a high prevalence of the disease. Of substantial clinical importance despite the syndrome of leptospiral pulmonary haemorrhage has emerged in recent years, in diverse places around the world.
Two important issues continue to confront clinicians regarding leptospirosis. The first is how to reliably establish the diagnosis. The most common way to diagnose leptospirosis is through serological tests either the Microscopic Agglutination Test (MAT) which detects serovar-specific antibodies, or a solid- phase assay for the detection of Immunoglobulin M (IgM) antibodies. Leptospira are present in the blood until they are cleared after 4-7 days following the production of Leptospira-specific antibodies, initially mainly of the IgM class. However, the greatest drawback of IgM detection assays is that IgM antibodies can persist for many months raising the questions about whether a positive IgM result accurately identifies a current infection.
The MAT is the cornerstone of the serodiagnosis for leptospirosis, because this assay has a high sensitivity and allows for the detection of group specific antibodies. Two major disadvantages of this test are that in regions where leptospirosis is common, there may be a substantial proportion of the population with elevated titres of MAT and secondly, the performance of MAT is restricted to laboratories that are capable of maintaining strains for the preparation of live antigens. Therefore, serological tests remain suboptimal for clinical use in diagnosing leptospirosis as depicted in Table 1. The most promising diagnostic methods are those that demonstrate the presence of the organisms.
Culture of Leptospira is difficult for a variety of reasons. The process is very laborious and can take up to three months. Therefore, isolation and culture are primarily used for retrospective diagnosis. Moreover, to culture the organism from tissues or body fluids, knowledge of the stage of infection is critical. In the acute phase, which lasts for about 10 days, the leptospires can often be cultured from blood or cerebrospinal fluid (CSF). Usually, when a specific antibody response is detected (at approximately 10 days), leptospires disappear from the blood. During the second phase, which may last up to several months, bacteriuria is often intermittent.
Molecular techniques to detect the presence of leptospiral DNA in blood, urine or spinal fluid have shown to be sensitive and specific; Sensitive assay for the detection of Leptospira DNA that is based upon amplification of the Leptospira’s (16S) gene have been developed. The data suggest that the PCR assay can be used on biological samples such as CSF, urine, or blood as a diagnostic tool for cases of suspected leptospirosis. The use of this technique is precluded by the cost and technical factors in non-reference laboratories.
Fig 1. Leptospira spp. under dark field microscopy
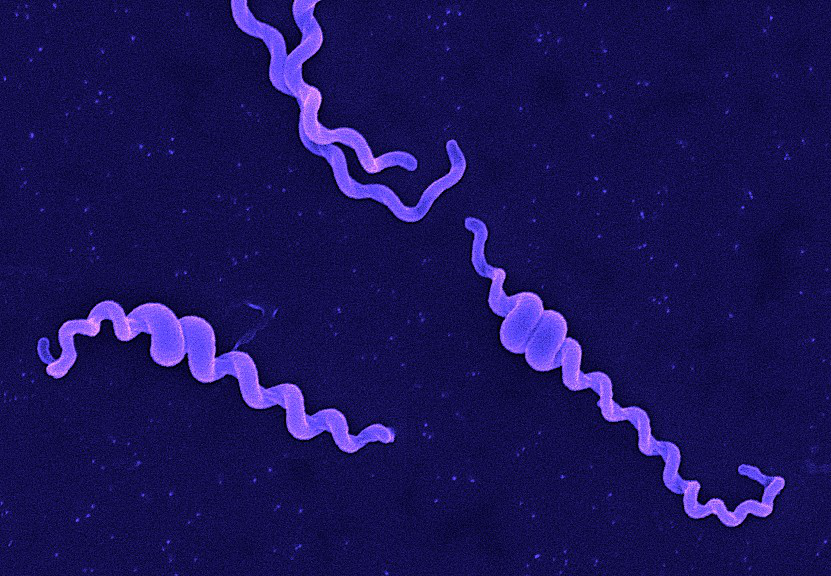 Table 1.
Table 1.
Advantages and disadvantages of diagnostic tests for the detection of Leptospirosis
| Test | Advantages | Disadvantages |
| Dark Field Microscopy (DFM) | Visualize Leptospirosis | Lack of sensitivity and specificity. 104 Leptospires/ml is necessary for one organism/field to be visible under DFM. |
| IgM ELISA | Most widely used | False positive, IgM cannot be detected in early stages of infection and can persist in blood for years. |
| Microscopic Agglutination Test (MAT) | Gold Standard | Less sensitive in early phase of disease. Labor intensive and complicated procedure as there is a need to maintain Leptospira strain for preparing liveantigen. |
| Polymerase Chain Reaction (PCR) | Successful in detecting Leptospira DNA in serum and urine samples of patients | Expensive reagents Requires large quantity of DNA. Cannot identify the infecting serovar. |
References:
Abdoel TH, Houwers DJ, van Dongen AM et al (2011) Rapid test for the serodiagnosis of acute canine leptospirosis. Vet Microbiol 150 (1-2), 211-213 PubMed .
Adin CA & Cowgill LD (2000) Treatment and outcome of dogs with leptospirosis-36 cases (1990-1998). JAVMA 216 (3), 371-375 PubMed .
Amouriaux Merièn P, Perolat P, Branton G, Saint IG. Polymerase Chain Reaction for detection of Leptospira spp. In clinical samples. Journal of Clinical Microbiology. 1992;30:2219–24. [ PMC free article ] [ PubMed ]
Anderson JF, Miller DA, Post JE et al (1993) Isolation of Leptospira interrogans serovar grippotyphosa from the skin of a dog. JAVMA 203 (11), 1550-1551 PubMed .
André-Fontaine G (2006) Canine leptospirosis–do we have a problem? Vet Microbiol 117 (1), 19-24 PubMed .
Boutilier P, Carr A & Schulman RL (2003) Leptospirosis in dogs: a serologic survey and case series 1996-2001. Vet Ther 4 (4), 387-396 PubMed .
Brandão AP, Camargo ED, da Silva ED, Silva MV, Abrão RV. Macroscopic agglutination test for rapid diagnosis of human leptospirosis. J ClinMicrobiol. 1998;36:3138–42. [ PMC free article ] [ PubMed ]
Brown CA, Roberts AW, Miller MA et al (1996) Leptospira interrogans serovar grippotyphosa infection in dogs. JAVMA 209 (7), 1265-1267 PubMed .
Burriel AR, Dalley C, Woodward MJ (2003) Prevalence of leptospira species among farmed and domestic animals in Greece. Vet Rec 153 (5), 146-148 PubMed .
Burr P, Lunn K, Yam P (2009) Current perspectives on canine leptospirosis. In Practice 31 (3), 98-102 VetMedResource .
Chernukha YG, Shishkina ZS, Baryshev PM, Kokovin IL. The dynamics of IgM and IgG antibodies in leptospiral infection in man. Zentralbl. Bakteriol.Mikrobiol. Hyg. 1976;236:336–43. [ PubMed ]
Cumberland PC, Everard COR, Wheeler JG, Levett PN. Persistence of anti-leptospiralIg M, IgG and agglutinating antibodies in patients presenting with acute febrile illness in Barbados 1979-1989. Eur J Epidemiol. 2001;17:601–08. [ PubMed ]
Ebani VV, Cerri D, Poli A et al (2003) Prevalence of Leptospira and Brucella antibodies in wild boars (Sus scrofa) in Tuscany, Italy. J Wildl Dis 39 (3), 718-722 PubMed .
Fraune CK, Schweighauser A & Francey T (2013) Evaluation of the diagnostic value of serologic microagglutination testing and a polymerase chain reaction assay for diagnosis of acute leptospirosis in dogs in a referral center. J Am Vet Med Assoc 242 (10), 1373- 1380 PubMed .
Geisen V, Stengel C, Brem S et al (2007) Canine leptospirosis infections-clinical signs and outcome with different suspected Leptospira serogroups (42 cases). J Small Anim Pract 48 (6), 324-328 PubMed .
Ghneim GS, Viers JH, Chomel BB et al (2007) Use of a case-control study and geographic information systems to determine environmental and demographic risk factors for canine leptospirosis. Vet Res 38 (1), 37-50 PubMed .
Goldstein RE, Lin RC, Langston CE et al (2006) Influence of infecting serogroup on clinical features of leptospirosis in dogs. J Vet Intern Med 20 (3), 489-494 PubMed .
Hartmann K, Egberink H, Pennisi MG et al (2013) Leptospira species infection in cats: ABCD guidelines on prevention and management. J Feline Med Surg 15 (7), 576-581 PubMed .
Harkin KR, Roshto YM & Sullivan JT (2003) Clinical application of a polymerase chain reaction assay for diagnosis for leptospirosis in dogs. JAVMA 222 (9), 1224-1229 PubMed .
Harkin KR & Gartrell CL (1996) Canine leptospirosis in New Jersey and Michigan-17 cases (1990-1995). JAAHA 32 (6), 495-501 PubMed .
Kohn B, Steinicke K, Arndt G et al (2010) Pulmonary abnormalities in dogs with leptospirosis. J Vet Intern Med 24 (6), 1277-1282 PubMed .
Meeyam T, Tablerk P, Petchanok B et al (2006) Seroprevalence and risk factors associated with leptospirosis in dogs. Southeast Asian J Trop Med Public Health 37 (1), 148-153 PubMed .
Moore GE, Guptill LF, Glickman LW et al (2006) Canine leptospirosis, United States, 2002-2004. Emerg Infect Dise 12 (3), 501-503 PubMed .
Pike RM. Laboratory-associated infections: summary and analysis of 3921 cases. Health Lab. Sci. 1976;13:105–14. [ PubMed ]
Silva MV, Camargo ED, Batista LA, et al. Behaviour of specific IgM, IgG and IgA class antibodies in human leptospirosis during the acute phase of the disease and during convalescence. J. Trop. Med. Hyg. 1995;98:268– 72. [ PubMed ]
Steger-Lieb A, Gerber B, Nicolet J et al (1999) [An old disease with a new face: canine leptospirosis does not lose its relevance]. Schweiz Arch Tierheilkd 141 (11), 499-507 PubMed .
Sykes JE, Hartmann K, Lunn KF et al (2011) 2010 ACVIM small animal consensus statement on leptospirosis: diagnosis, epidemiology, treatment, and prevention. J Vet Intern Med 25 (1), 1-13 PubMed .
Turner LH. Leptospirosis I. Trans R. Soc Trop Med Hyg. 1967;61:842–55. [ PubMed ]
Turner LH. Leptospirosis II. Serology. Trans. R. Soc. Trop. Med.Hyg. 1968;62:880–89. [ PubMed ]
Ward MP, Guptill LF & Wu CC (2004) Evaluation of environmental risk factors for leptospirosis in dogs: 36 cases (1997-2002). JAVMA 225 (1), 72-77 PubMed .

