Maigan Espinili Maruquin
I. Characteristics / Epidemiology
The Bartonella spp. have wide distribution worldwide wherein antibody prevalence in cats which ranged from 8–53% was recorded in Europe (Pennisi, Marsilio et al. 2013, Zangwill 2013) while approximately 5-80% of cats worldwide were recorded of serological evidence on exposure to this bacteria (Guptill 2012). They cause wide range of clinical syndromes depending on the infecting species and immune status of the infected (Zangwill 2013).
The Bartonella are small and fastidious Gram-negative bacteria which are transmitted by arthropods and infect wide range of hosts including: human, rodents, rabbits, felids, canids, ruminants. However, cats are the primary mammalian reservoir and vector for transmission (Guptill 2012). The B. henselae is known as a common species to both cats and humans. This species also cause Cat Scratch Disease (CSD) to people. It is naturally transmitted between cats by the flea itself, Ctenocephalides felis felis, or the flea feces. The Bartonella stays in the red blood cells of infected cats and ingested by flea (Chomel, Kasten et al. 1996, Pennisi, Marsilio et al. 2013). While Bartonella persists in the environment in the flea faeces, it also amplifies the infection in the flea hindgut (Finkelstein, Brown et al. 2002). The feces of a contaminated flea, which are deposited in the skin, ends up under the cat’s claw from self- scratching (Chomel, Kasten et al. 1996, Pennisi, Marsilio et al. 2013). Moreover, the tick bites may also transmit B. henselae to humans (Lucey, Dolan et al. 1992, Klotz, Ianas et al. 2011, Biancardi and Curi 2014).
While B. henselae are the most commonly detected Bartonella infection in cats and approximately 10% B. clarridgeiae, other species were reported much less commonly. However, the prevalence of different genotypes of B henselae were recorded from regional differences, and are not limited to domestic cats (Guptill 2012).
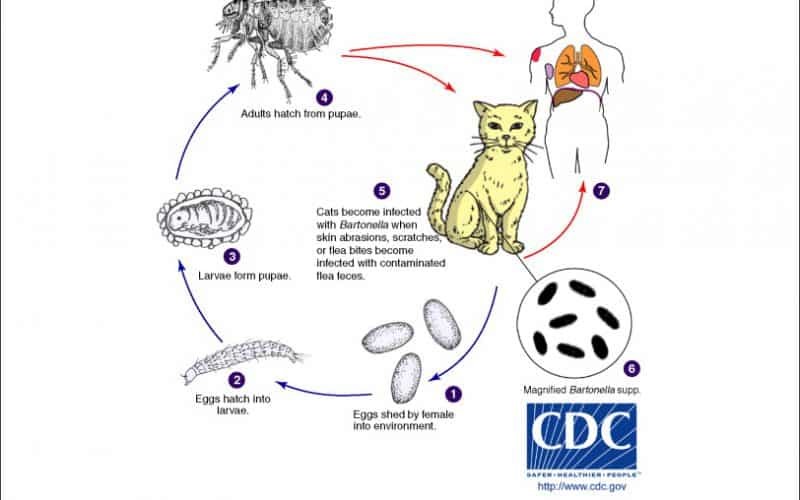
Fig. 01. The Life Cycle of Bartonella spp. (https://www.northcarolinahealthnews.org/2016/12/19/north-carolina-ranks-as-high-risk-zone-for-cat-scratch-disease/)
II. Pathogenesis/ Clinical Signs
It has been described that B. henselae were transmitted by cat fleas of infected cats to non- infected cats (Chomel, Kasten et al. 1996), or by intradermal inoculation of cats with flea excrement wherein contamination of skin wounds with flea excrement occurs (Finkelstein, Brown et al. 2002). While the infection caused by Bartonella depends on the species and the host immunity (Cunningham and Koehler 2000), infections may cause necrosis with histiocytes, lymphocytes, and giant cells, forming a granuloma for immunocompetent patients (LeBoit PE, 1997) (Biancardi and Curi 2014).
Although cats can generate antibody and cell-mediated immune responses against Bartonella infections, the species B. henselae and B. clarridgeiae are commonly chronic and relapsing. On experimentally infected cats, most were clinically normal, while severity relies on the various strains used for inoculation. Abscess were observed on the inoculation sites on cats inoculated intradermally including localized peripheral lymphadenomegaly, short periods of fever (Guptill 2012), mild neurological signs and reproductive failure (Kordick, Brown et al. 1999). On the other hand, cats who received higher doses of B. henselae, despite remaining responsive, showed lethargy, fever, partial anorexia and enlarged lymph nodes (Guptill, Slater et al. 1997, Stützer and Hartmann 2012).
Meanwhile, B. henselae naturally infected cats do not show clinical signs (Stützer and Hartmann 2012). However, Bartonella infection was suggested to be associated in chronic gingivostomatitis, but antibodies or organisms’ prevalence in diseased cats were lower (Ueno, Hohdatsu et al. 1996, Glaus, Hofmann-Lehmann et al. 1997, Quimby, Elston et al. 2008, Pennisi, La Camera et al. 2009, Belgard, Truyen et al. 2010, Dowers, Hawley et al. 2010, Namekata, Kasten et al. 2010, Pennisi, Marsilio et al. 2013). Also, there were few cases on B. henselae-associated endocarditis or myocarditis (Chomel, Wey et al. 2003, Chomel, Kasten et al. 2009). The B. henselae, however may be of importance in immune complex diseases in cats wherein a strong correlation between the presence of antibodies against Bartonella species and hyperglobulinaemia was reported (Whittemore, Hawley et al. 2012).
III. Diagnosis
Having been exposed to fleas, cats with such history, aside from having clinical signs of Batonella infection, shall be tested for possible Bartonella infection (Guptill 2012). Laboratory testing shall be required for feline blood donors owned by immunosuppressed person or when a human with Bartonella-related disease is in the cat’s home (Pennisi, Marsilio et al. 2013).
While isolation of the bacterium is considered the gold standard, a positive culture is not confirmatory (Pennisi, Marsilio et al. 2013). The relapsing nature of Bartonella bacteraemia makes the blood culture not so sensitive to diagnose. Thus, this tool is suggested for sick cats with history and clinical presentation of possible infection of Bartonella (Guptill 2012). Therefore, diagnosis is by exclusion, and by assessing the response to therapy (Pennisi, Marsilio et al. 2013).
Blood samples, aqueous humour, cerebrospinal fluid or tissues, and several gene targets may be used for PCR (Pennisi, Marsilio et al. 2013). Although standard PCR may be no more sensitive than blood culture, Real Time PCR may have better sensitivity (Valasek and Repa 2005, Kamrani, Parreira et al. 2008, Guptill 2012). The PCR products may be sequenced, which may lead to identification of Bartonella species (Guptill 2012).
Serology tests using immunofluorescent antibody (IFAT), enzyme-linked immunosorbent assay (ELISA), and western blot tests are also available (Guptill 2012). However, these are considered to be more useful in exclusion rather than confirmation (Chomel BB, et al., 1995)(Gurfield, Boulouis et al. 2001, Fabbi, De Giuli et al. 2004, Bennett, Gunn-Moore et al. 2011, Pennisi, Marsilio et al. 2013).
IV. Treatment and Management
There are several drugs that were used to Bartonella infected cats including doxycycline, amoxicillin, amoxicillin–clavulanic acid, enrofloxacin, erythromycin, rifampicin (Greene, McDermott et al. 1996, Regnery, Rooney et al. 1996, Kordick and Breitschwerdt 1997). However, no definite elimination of Bartonella infection in cats by antibiotic treatments was reported but doxycycline may be a good initial antibiotic choice wherein higher doses given for a longer time appears to be more effective (Guptill 2012).
A household with immunocompromised people or with children should have their infected cats treated, whether or not they show clinical signs. Whereas, treatment is also recommended when Bartonella species actually caused disease in a cat (Stützer and Hartmann 2012).
Transmission of B. henselae is likely to occur from infected cats to humans through contamination of scratches or other skin abrasions with flea excrement containing B. henselae. Therefore, precautions are advised: vector control, avoiding interactions with cats that result in scratches or bites, supervising children’s interactions with cats, thoroughly washing bite or scratch wounds, and acquiring new pets of known good health status that are and have been ectoparasite-free (Kaplan et al, 2009)(Guptill 2012).
References
LeBoit PE. Bacillary angiomatosis. In Connor DH, Chandler FW, Manz HJ, et al. (eds.), Pathology of infectious diseases. Stamford, CT: Appleton & Lange; 1997: 239–252.
Chomel BB, Gurfield AN, Boulouis HJ, Kasten RW and Piemont Y. Réservoir félin de l’agent de la maladie des griffes du chat, Bartonella henselae, en région parisienne: resultants préliminaires. Rec Med Vet 1995; 171: 841–845.
KAPLAN, A. J., BENSON, C., HOLMES, K. K., BROOKS, J. T., PAU, A. & MASUR, H. (2009) Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Morbidity and Mortality Weekly Report 58, 1-198.
Belgard, S., U. Truyen, J. C. Thibault, C. Sauter-Louis and K. Hartmann (2010). “Relevance of feline calicivirus, feline immunodeficiency virus, feline leukemia virus, feline herpesvirus and Bartonella henselae in cats with chronic gingivostomatitis.” Berl Munch Tierarztl Wochenschr 123(9-10): 369-376.
Bennett, A. D., D. A. Gunn-Moore, M. Brewer and M. R. Lappin (2011). “Prevalence of Bartonella species, haemoplasmas and Toxoplasma gondii in cats in Scotland.” J Feline Med Surg 13(8): 553-557.
Biancardi, A. L. and A. L. L. Curi (2014). “Cat-Scratch Disease.” Ocular Immunology and Inflammation 22(2): 148-154.
Chomel, B. B., R. W. Kasten, K. Floyd-Hawkins, B. Chi, K. Yamamoto, J. Roberts-Wilson, A. N. Gurfield, R. C. Abbott, N. C. Pedersen and J. E. Koehler (1996). “Experimental transmission of Bartonella henselae by the cat flea.” J Clin Microbiol 34(8): 1952-1956.
Chomel, B. B., R. W. Kasten, C. Williams, A. C. Wey, J. B. Henn, R. Maggi, S. Carrasco, J. Mazet, H. J. Boulouis, R. Maillard and E. B. Breitschwerdt (2009). “Bartonella endocarditis: a pathology shared by animal reservoirsand patients.” Ann N Y Acad Sci 1166: 120-126.
Chomel, B. B., A. C. Wey, R. W. Kasten, B. A. Stacy and P. Labelle (2003). “Fatal case of endocarditis associated with Bartonella henselae type I infection in a domestic cat.” J Clin Microbiol 41(11): 5337-5339.
Cunningham, E. T. and J. E. Koehler (2000). “Ocular bartonellosis.” Am J Ophthalmol 130(3): 340-349.
Dowers, K. L., J. R. Hawley, M. M. Brewer, A. K. Morris, S. V. Radecki and M. R. Lappin (2010). “Association of Bartonella species, feline calicivirus, and feline herpesvirus 1 infection with gingivostomatitis in cats.” J Feline Med Surg 12(4): 314-321.
Fabbi, M., L. De Giuli, M. Tranquillo, R. Bragoni, M. Casiraghi and C. Genchi (2004). “Prevalence of Bartonella henselae in Italian stray cats: evaluation of serology to assess the risk of transmission of Bartonella to humans.” J Clin Microbiol 42(1): 264-268.
Finkelstein, J. L., T. P. Brown, K. L. O’Reilly, J. Wedincamp, Jr. and L. D. Foil (2002). “Studies on the growth of Bartonella henselae in the cat flea (Siphonaptera: Pulicidae).” J Med Entomol 39(6): 915-919.
Glaus, T., R. Hofmann-Lehmann, C. Greene, B. Glaus, C. Wolfensberger and H. Lutz (1997). “Seroprevalence of Bartonella henselae infection and correlation with disease status in cats in Switzerland.” Journal of clinical microbiology 35(11): 2883-2885.
Greene, C. E., M. McDermott, P. H. Jameson, C. L. Atkins and A. M. Marks (1996). “Bartonella henselae infection in cats: evaluation during primary infection, treatment, and rechallenge infection.” Journal of clinical microbiology 34(7): 1682-1685.
Guptill, L. (2012). “Bartonella infections in cats: what is the significance?” In Practice 34(8): 434.
Guptill, L., L. Slater, C. C. Wu, T. L. Lin, L. T. Glickman, D. F. Welch and H. HogenEsch (1997). “Experimental infection of young specific pathogen-free cats with Bartonella henselae.” J Infect Dis 176(1): 206-216.
Gurfield, A. N., H. J. Boulouis, B. B. Chomel, R. W. Kasten, R. Heller, C. Bouillin, C. Gandoin, D. Thibault, C. C. Chang, F. Barrat and Y. Piemont (2001). “Epidemiology of Bartonella infection in domestic cats in France.” Vet Microbiol 80(2): 185-198.
Kamrani, A., V. R. Parreira, J. Greenwood and J. F. Prescott (2008). “The prevalence of Bartonella, hemoplasma, and Rickettsia felis infections in domestic cats and in cat fleas in Ontario.” Canadian journal of veterinary research = Revue canadienne de recherche veterinaire 72(5): 411-419.
Klotz, S. A., V. Ianas and S. P. Elliott (2011). “Cat-scratch Disease.” Am Fam Physician 83(2): 152-155.
Kordick, D. L. and E. B. Breitschwerdt (1997). “Relapsing bacteremia after blood transmission of Bartonella henselae to cats.” Am J Vet Res 58(5): 492-497.
Kordick, D. L., T. T. Brown, K. Shin and E. B. Breitschwerdt (1999). “Clinical and pathologic evaluation of chronic Bartonella henselae or Bartonella clarridgeiae infection in cats.” Journal of clinical microbiology 37(5): 1536-1547.
Lucey, D., M. J. Dolan, C. W. Moss, M. Garcia, D. G. Hollis, S. Wegner, G. Morgan, R. Almeida, D. Leong, K. S. Greisen and et al. (1992). “Relapsing illness due to Rochalimaea henselae in immunocompetent hosts: implication for therapy and new epidemiological associations.” Clin Infect Dis 14(3): 683-688.
Namekata, D. Y., R. W. Kasten, D. A. Boman, M. H. Straub, L. Siperstein-Cook, K. Couvelaire and B. B. Chomel (2010). “Oral shedding of Bartonella in cats: correlation with bacteremia and seropositivity.” Veterinary microbiology 146(3-4): 371-375.
Pennisi, M. G., E. La Camera, L. Giacobbe, B. M. Orlandella, V. Lentini, S. Zummo and M. T. Fera (2009). “Molecular detection of Bartonella henselae and Bartonella clarridgeiae in clinical samples of pet cats from Southern Italy.” Research in veterinary science 88: 379-384.
Pennisi, M. G., F. Marsilio, K. Hartmann, A. Lloret, D. Addie, S. Belák, C. Boucraut-Baralon, H. Egberink, T. Frymus, T. Gruffydd-Jones, M. J. Hosie, H. Lutz, K. Möstl, A. D. Radford, E. Thiry, U. Truyen and M. C. Horzinek (2013). “Bartonella Species Infection in Cats: ABCD guidelines on prevention and management.” Journal of Feline Medicine and Surgery 15(7): 563-569.
Quimby, J. M., T. Elston, J. Hawley, M. Brewer, A. Miller and M. R. Lappin (2008). “Evaluation of the association of Bartonella species, feline herpesvirus 1, feline calicivirus, feline leukemia virus and feline immunodeficiency virus with chronic feline gingivostomatitis.” J Feline Med Surg 10(1): 66-72.
Regnery, R. L., J. A. Rooney, A. M. Johnson, S. L. Nesby, P. Manzewitsch, K. Beaver and J. G. Olson (1996). “Experimentally induced Bartonella henselae infections followed by challenge exposure and antimicrobial therapy in cats.” Am J Vet Res 57(12): 1714-1719.
Stützer, B. and K. Hartmann (2012). “Chronic Bartonellosis in Cats: What are the potential implications?” Journal of Feline Medicine and Surgery 14(9): 612-621.
Ueno, H., T. Hohdatsu, Y. Muramatsu, H. Koyama and C. Morita (1996). “Does coinfection of Bartonella henselae and FIV induce clinical disorders in cats?” Microbiol Immunol 40(9): 617-620.
Valasek, M. A. and J. J. Repa (2005). “The power of real-time PCR.” Adv Physiol Educ 29(3): 151-159.
Whittemore, J. C., J. R. Hawley, S. V. Radecki, J. D. Steinberg and M. R. Lappin (2012). “Bartonella species antibodies and hyperglobulinemia in privately owned cats.” Journal of veterinary internal medicine 26(3): 639-644.
Zangwill, K. M. (2013). Cat Scratch Disease and Other Bartonella Infections. Hot Topics in Infection and Immunity in Children IX. N. Curtis, A. Finn and A. J. Pollard. New York, NY, Springer New York: 159-166.

