Maigan Espinili Maruquin
Structure and Replication
The Caliciviruses are small, non-enveloped, positive strand RNA viruses which infects a wide range of hosts (Sosnovtsev and Green 2003), which, in cats, feline calicivirus- (FCV) is associated with upper respiratory tract infections (Gaskell, R. M., 1985).
The FCV is a naked and icosaedral virus, 30–40 nm in diameter (Carter MJ, Madeley CR, 1987). The single protein capsid is 65- 66 kDa (Neill, Reardon et al. 1991, Carter, Milton et al. 1992), having a single-stranded positive- sense RNA genome of 7.7 kb (Carter, Milton et al. 1992). Having 3 ORFs, the ORF1 encodes a 200 kDa polyprotein to be processed into six mature non-structural proteins (Di Martino, Marsilio et al. 2007). Whereas, the ORF2, which is divided into six regions (A, B, C, D, E, and F), encodes a 73 kDa capsid precursor (preVP1) which undergoes rapid proteolytic cleavage during the maturation process, yielding a mature 60 kDa capsid protein (VP1) (Carter 1989, Carter, Milton et al. 1992, Sosnovtsev, Sosnovtseva et al. 1998, Sosnovtsev and Green 2003, Di Martino, Marsilio et al. 2007, Prikhodko, Sandoval-Jaime et al. 2014). The E-region is responsible for antigenic properties of the FCV virion (Guiver, Littler et al. 1992, Milton, Turner et al. 1992, Seal, Ridpath et al. 1993, Tohya, Yokoyama et al. 1997, Prikhodko, Sandoval-Jaime et al. 2014) and is also responsible to the protruding (P) subdomain P2 on the virion surface (Chen, Neill et al. 2006, Ossiboff, Zhou et al. 2010, Prikhodko, Sandoval-Jaime et al. 2014). Finally, the ORF3, encodes a small minor structural protein, 12 kDa, with 106 amino acids, VP2, and is essential for the production of infectious virions (Sosnovtsev, Belliot et al. 2005, Di Martino, Marsilio et al. 2007). The replication of FCV includes a plus-sense genomic RNA of approximately 7.7 kb, and a subgenomic RNA of 2.4 kb (Herbert, Brierley et al. 1996, Sosnovtsev and Green 2003)
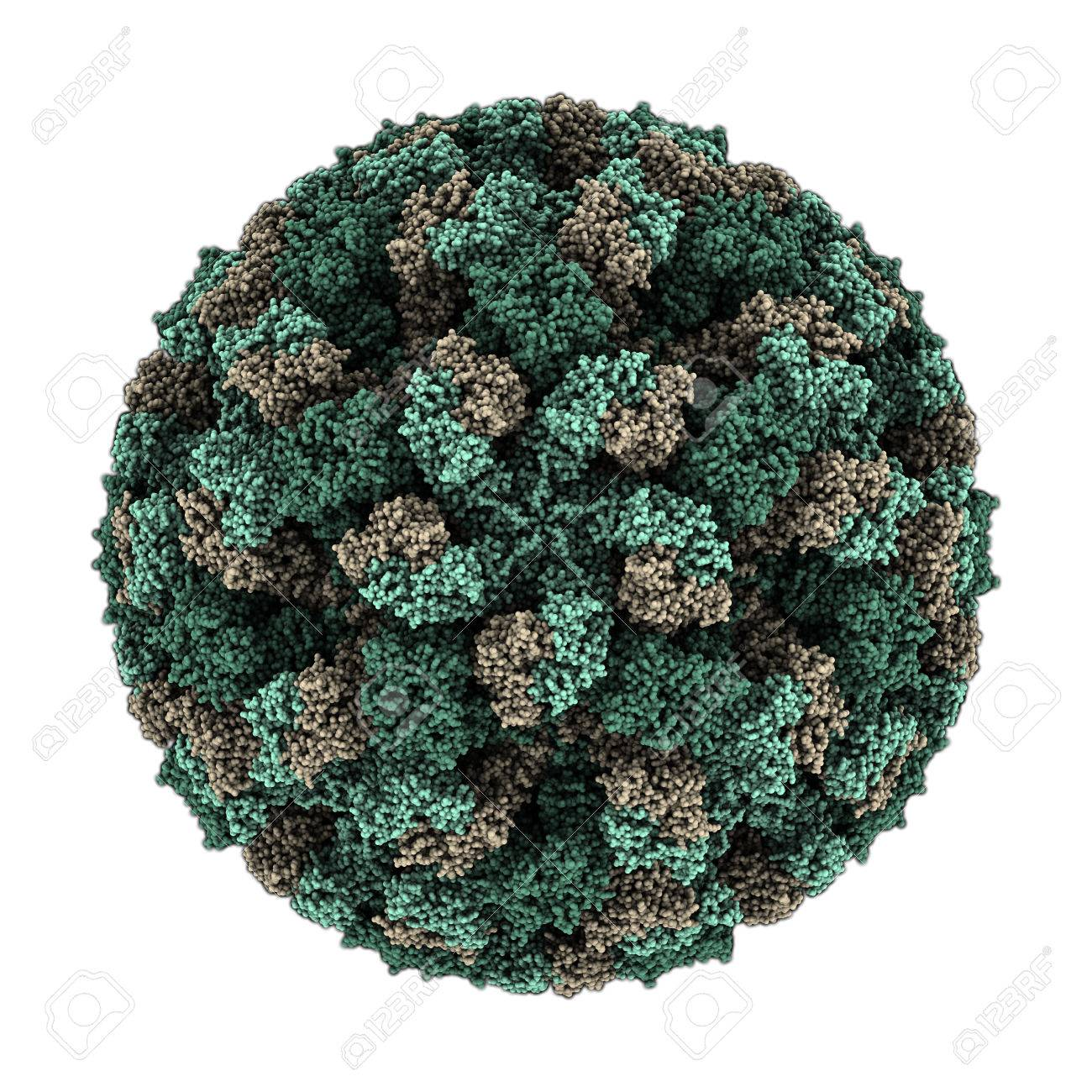
Fig. 01. The Feline Calicivirus
Epidemiology
The FCV has originally been isolated from the gastrointestinal tract of cats in New Zealand (Fastier 1957, Pesavento, Chang et al. 2008). High viral prevalence in a population has been explained by long-term shedders (Hurley, Pesavento et al. 2004), sequential infections and episodes of reinfection (Coyne, Gaskell et al. 2007, Pesavento, Chang et al. 2008). With the features of FCV: high genetic variability, a capacity to persist in infected individuals, stability in the environment, and ubiquity in feline populations worldwide are causing the severity of the disease (Pesavento, Chang et al. 2008).
Humans are not susceptible to FCV (Radford, Addie et al. 2009). Cats may also shed from 30 days to years even after recovery (Wardley 1976). The FCV infection is widespread among cat populations, having a report of 25–40% from colonies and shelters (Wardley, Gaskell et al. 1974, Bannasch and Foley 2005, Helps, Lait et al. 2005, Radford, Addie et al. 2009).
Pathogenesis / Clinical Signs
The FCV enters via nasal, oral or conjunctival routes wherein oropharynx is the primary site of replication. At 3 to 4 days of post infection, transient viraemia occurs and can be detected in many other tissues. Epithelial necrosis occurs while vesicles develop into ulcers, which is usually in the margin of the tongue. Neutrophils infiltrate the dermis of other tissues; and it takes 2 to 3 weeks to start healing (Gaskell RM, 2006) (Radford, Coyne et al. 2007, Radford, Addie et al. 2009).
a. Oral and Upper Respiratory Tract Disease
Mostly in kittens, incubation period takes 2–10 days. oral ulceration, sneezing and serous nasal discharge start to appear (Gaskell RM, 2006) (Radford, Addie et al. 2009). In some severe cases, pneumonia occurs, pulmonary lesions occur more rarely, dyspnoea, coughing, fever and depression, can occur, particularly in young kittens (Radford, Coyne et al. 2007, Radford, Addie et al. 2009).
b. FCV-associated Lameness
There could be lesions in the joints of cats with acute synovitis, consists with thickening of the synovial membrane and an increase in quantity of synovial fluid (Dawson, Bennett et al. 1994, Radford, Coyne et al. 2007). Fever could be observed following FCV infection and vaccination, usually a few days or weeks after the acute oral or respiratory signs (Pedersen NC, 1983) (Radford, Addie et al. 2009).
c. Virulent systemic FCV disease (VSD)
The virulent systemic FCV disease is manifested by systemic inflammatory response syndrome, disseminated intra – vascular coagulation, multi- organ failure and death, with mortality rates of up to 67% (Foley, Hurley et al. 2006, Radford, Addie et al. 2009). During VSD, virus infiltrates cellular compartments not normally associated with FCV; lesions are widespread with ulceration of the skin, broncho-interstitial pneumonia and necrosis in the liver, spleen and pancreas (Pedersen, Elliott et al. 2000, Pesavento, Maclachlan et al. 2004, Radford, Coyne et al. 2007). The FCV evolves to be efficiently transmitted among the cat population (Radford, Coyne et al. 2007). Clinical signs initially appear as a severe acute upper respiratory tract disease. Lesions, ulcers and alopecia are observed on the nose, lips and ears, around the eyes and on the footpads (Radford, Addie et al. 2009).
d. Molecular pathogenesis
Cytopathic effect is observed in infected cells, with cell rounding and membrane blebbing (Knowles J.O, 1988), which leads to inhibition of cellular protein synthesis (shut-off) (Willcocks, Carter et al. 2004) and may stop translation of cellular mRNAs to focus on the viral VpG-bound RNA (Radford, Coyne et al. 2007). It has been identified that the junctional adhesion molecule-1 (JAM-1) serves as a cellular receptor for FCV in cell culture (Makino, Shimojima et al. 2006).
Diagnosis
Nucleic acid can be detected in conjunctival and oral swabs, blood, skin scrapings and lung tissue using conventional, nested and real-time reverse-transcriptase PCR (RT-PCR) assays. Molecular assays are validated in a large panel of strains to minimize false-negative results. The use of reverse transcriptase PCR allows unique virus strains detection (Abd-Eldaim, Potgieter et al. 2005, Radford, Addie et al. 2009).
The presence of replicating virus can be isolated from nasal, conjunctival and oropharyngeal swabs, depending on the numbers of infectious virions in the sample, virus inactivation during transit, or the presence of antibodies in the sample (Gaskell R, 1998). The virus isolation is less sensitive to strain variation than RT-PCR (Radford, Addie et al. 2009).
The ELISA can be used to detect FCV antibodies, although sero- prevalence is high due to natural infection and vaccination. While the VNA titres can be useful to predict whether or not a cat is protected (Radford, Addie et al. 2009).
Vaccination
There are several vaccines currently available against FCV, based on whole viral antigens grown in cell culture. There are available monovalent or single- strain based, bivalent, live-attenuated and inactivated (both adjuvanted and non-adjuvanted) vaccines. Recommendations for vaccination is with a primary course at 8–9 and 12 weeks followed by annual boosters (Radford, Coyne et al. 2007).
However, vaccination against FCV, despite providing good protection against acute oral and upper respiratory tract disease in most cases, doesn’t prevent infection or shedding. (Southerden and Gorrel 2007). Also, no vaccine has been reported to protect against all FCV field strains (Radford, Addie et al. 2009). Although vaccinating pregnant queens may reduce disease in young kittens by boosting their MDA (Iglauer F, 1989), safety of vaccines in pregnant queens is largely unknown (Radford, Coyne et al. 2007).
Disease Management and Treatment
In the case of dehydration and restoration of electrolyte and acid–base disturbances, intravenous fluid administration is required. Fever and oral pain may be treated with nonsteroidal anti-inflammatory drugs, while food may be blended since infected cats may refuse to eat. In the case that the cat refused to eat for three days or more, placement of a feeding tube and enteral nutrition is indicated. Further, antibiotics that achieve good penetration into the respiratory tract and/or oral mucosa from your veterinarian will be administered for severe disease and suspected bacterial infection (Radford, Addie et al. 2009). Vaccination may be sufficient for individual household cats, however, in boarding and rescue catteries, minimizing viral loads and the spread of virus through the population is critical thus, preventive measures shall be implemented like vaccination, avoiding overcrowding and good husbandry/hygiene (Radford, Coyne et al. 2007).
The awareness on the disease of the cat owners and shelters’ staff is very important. Shedding of the virus from the FCV infected cats are likely from their respiratory and oral secretions, or from skin may be highly infectious. Isolations were also collected from faeces and urine, therefore, areas and equipment require disinfection (Radford, Coyne et al. 2007).
Severe infection with VSD needs intensive supportive therapy (fluid therapy, antibiotics) plus steroids and interferon (Hurley KF, 2006)(Radford, Addie et al. 2009). Although antiviral agents, like Ribavirin, were able to inhibit FCV replication in vitro, side effects and toxicity were reported (Povey 1978, Radford, Addie et al. 2009).
References
- Hurley KF. Virulent calicivirus infection in cats. Proceedings of the 24th Annual American College of Veterinary Internal Medicine Forum; 2006, May 31– June 3; 585.
- Iglauer F., Gartner K., Morstedt R., Maternal protection against feline respiratory disease by means of booster vaccinations during pregnancy – a retrospective clinical study, Kleintierpraxis (1989) 34:235.
- Gaskell R, Dawson S. Feline respiratory disease. In: Greene CE, ed. Infectious diseases of the dog and cat. Philadelphia: WB Saunders Company, 1998: 97–106
- Knowles J.O., Studies on feline calicivirus with particular reference to chronic stomatitis in the cat, 1988, University of Liverpool.
- Pedersen NC, Laliberte L, Ekman S. A transient febrile ‘limping’ syndrome of kittens caused by two different strains of feline calicivirus. Feline Pract 1983; 13: 26–35.
- Gaskell RM, Dawson S, Radford AD. Feline respiratory disease. In: Greene CE, ed. Infectious diseases of the dog and cat. Philadelphia: Saunders Elsevier, 2006: 145–54.
- Gaskell, R. M. (1985). Viral-induced upper respiratory tract diseases. In Feline Medicine and Therapeutics, edited by E. A. Chandler, C. J. Gaskell and A. D. R. Hilbery, pp. 257-70. Oxford: Blackwell Scientific Publications.
- Carter MJ, Madeley CR (1987) Caliciviridae. In: Nennut MV, Steven AC (eds) Animal virus structure. Elsevier, Amsterdam, pp 99, 121–128 (Perspectives in Medicine and Virology, vol 3)
- Abd-Eldaim, M., L. Potgieter and M. Kennedy (2005). “Genetic analysis of feline caliciviruses associated with a hemorrhagic-like disease.” J Vet Diagn Invest 17(5): 420-429.
- Bannasch, M. J. and J. E. Foley (2005). “Epidemiologic evaluation of multiple respiratory pathogens in cats in animal shelters.” J Feline Med Surg 7(2): 109-119.
- Carter, M. J. (1989). “Feline calicivirus protein synthesis investigated by western blotting.” Arch Virol 108(1-2): 69-79.
- Carter, M. J., I. D. Milton, J. Meanger, M. Bennett, R. M. Gaskell and P. C. Turner (1992). “The complete nucleotide sequence of a feline calicivirus.” Virology 190(1): 443-448.
- Carter, M. J., I. D. Milton, P. C. Turner, J. Meanger, M. Bennett and R. M. Gaskell (1992). “Identification and sequence determination of the capsid protein gene of feline calicivirus.” Arch Virol 122(3-4): 223-235.
- Chen, R., J. D. Neill, M. K. Estes and B. V. V. Prasad (2006). “X-ray structure of a native calicivirus: Structural insights into antigenic diversity and host specificity.” Proceedings of the National Academy of Sciences 103(21): 8048.
- Coyne, K. P., R. M. Gaskell, S. Dawson, C. J. Porter and A. D. Radford (2007). “Evolutionary mechanisms of persistence and diversification of a calicivirus within endemically infected natural host populations.” J Virol 81(4): 1961-1971.
- Dawson, S., D. Bennett, S. D. Carter, M. Bennett, J. Meanger, P. C. Turner, M. J. Carter, I. Milton and R. M. Gaskell (1994). “Acute arthritis of cats associated with feline calicivirus infection.” Res Vet Sci 56(2): 133-143.
- Di Martino, B., F. Marsilio and P. Roy (2007). “Assembly of feline calicivirus-like particle and its immunogenicity.” Veterinary Microbiology 120(1): 173-178.
- Fastier, L. B. (1957). “A new feline virus isolated in tissue culture.” Am J Vet Res 18(67): 382-389.
- Foley, J., K. Hurley, P. A. Pesavento, A. Poland and N. C. Pedersen (2006). “Virulent systemic feline calicivirus infection: local cytokine modulation and contribution of viral mutants.” J Feline Med Surg 8(1): 55-61.
- Guiver, M., E. Littler, E. O. Caul and A. J. Fox (1992). “The cloning, sequencing and expression of a major antigenic region from the feline calicivirus capsid protein.” Journal of General Virology 73(9): 2429-2433.
- Helps, C. R., P. Lait, A. Damhuis, U. Björnehammar, D. Bolta, C. Brovida, L. Chabanne, H. Egberink, G. Ferrand, A. Fontbonne, M. G. Pennisi, T. Gruffydd-Jones, D. Gunn-Moore, K. Hartmann, H. Lutz, E. Malandain, K. Möstl, C. Stengel, D. A. Harbour and E. A. Graat (2005). “Factors associated with upper respiratory tract disease caused by feline herpesvirus, feline calicivirus, Chlamydophila felis and Bordetella bronchiseptica in cats: experience from 218 European catteries.” Vet Rec 156(21): 669-673.
- Herbert, T. P., I. Brierley and T. D. Brown (1996). “Detection of the ORF3 polypeptide of feline calicivirus in infected cells and evidence for its expression from a single, functionally bicistronic, subgenomic mRNA.” J Gen Virol 77 ( Pt 1): 123-127.
- Hurley, K. E., P. A. Pesavento, N. C. Pedersen, A. M. Poland, E. Wilson and J. E. Foley (2004). “An outbreak of virulent systemic feline calicivirus disease.” J Am Vet Med Assoc 224(2): 241-249.
- Makino, A., M. Shimojima, T. Miyazawa, K. Kato, Y. Tohya and H. Akashi (2006). “Junctional adhesion molecule 1 is a functional receptor for feline calicivirus.” J Virol 80(9): 4482-4490.
- Milton, I. D., J. Turner, A. Teelan, R. Gaskell, P. C. Turner and M. J. Carter (1992). “Location of monoclonal antibody binding sites in the capsid protein of feline calicivirus.” Journal of General Virology 73(9): 2435-2439.
- Neill, J. D., I. M. Reardon and R. L. Heinrikson (1991). “Nucleotide sequence and expression of the capsid protein gene of feline calicivirus.” Journal of virology 65(10): 5440-5447.
- Ossiboff, R. J., Y. Zhou, P. J. Lightfoot, B. V. Prasad and J. S. Parker (2010). “Conformational changes in the capsid of a calicivirus upon interaction with its functional receptor.” J Virol 84(11): 5550-5564.
- Pedersen, N. C., J. B. Elliott, A. Glasgow, A. Poland and K. Keel (2000). “An isolated epizootic of hemorrhagic-like fever in cats caused by a novel and highly virulent strain of feline calicivirus.” Vet Microbiol 73(4): 281-300.
- Pesavento, P. A., K.-O. Chang and J. S. L. Parker (2008). “Molecular Virology of Feline Calicivirus.” Veterinary Clinics of North America: Small Animal Practice 38(4): 775-786.
- Pesavento, P. A., N. J. Maclachlan, L. Dillard-Telm, C. K. Grant and K. F. Hurley (2004). “Pathologic, Immunohistochemical, and Electron Microscopic Findings in Naturally Occurring Virulent Systemic Feline Calicivirus Infection in Cats.” Veterinary Pathology 41(3): 257-263.
- Povey, R. C. (1978). “Effect of orally administered ribavirin on experimental feline calicivirus infection in cats.” Am J Vet Res 39(8): 1337-1341.
- Prikhodko, V. G., C. Sandoval-Jaime, E. J. Abente, K. Bok, G. I. Parra, I. B. Rogozin, E. N. Ostlund, K. Y. Green and S. V. Sosnovtsev (2014). “Genetic characterization of feline calicivirus strains associated with varying disease manifestations during an outbreak season in Missouri (1995–1996).” Virus Genes 48(1): 96-110.
- Radford, A. D., D. Addie, S. Belák, C. Boucraut-Baralon, H. Egberink, T. Frymus, T. Gruffydd-Jones, K. Hartmann, M. J. Hosie, A. Lloret, H. Lutz, F. Marsilio, M. G. Pennisi, E. Thiry, U. Truyen and M. C. Horzinek (2009). “Feline Calicivirus Infection: ABCD Guidelines on Prevention and Management.” Journal of Feline Medicine and Surgery 11(7): 556-564.
- Radford, A. D., K. P. Coyne, S. Dawson, C. J. Porter and R. M. Gaskell (2007). “Feline calicivirus.” Vet Res 38(2): 319-335.
- Seal, B. S., J. F. Ridpath and W. L. Mengeling (1993). “Analysis of feline calicivirus capsid protein genes: identification of variable antigenic determinant regions of the protein.” J Gen Virol 74 ( Pt 11): 2519-2524.
- Sosnovtsev, S. V., G. Belliot, K. O. Chang, O. Onwudiwe and K. Y. Green (2005). “Feline calicivirus VP2 is essential for the production of infectious virions.” J Virol 79(7): 4012-4024.
- Sosnovtsev, S. V. and K. Y. Green (2003). IV, 2. Feline calicivirus as a model for the study of calicivirus replication. Perspectives in Medical Virology, Elsevier. 9: 467-488.
- Sosnovtsev, S. V., S. A. Sosnovtseva and K. Y. Green (1998). “Cleavage of the feline calicivirus capsid precursor is mediated by a virus-encoded proteinase.” J Virol 72(4): 3051-3059.
- Southerden, P. and C. Gorrel (2007). “Treatment of a case of refractory feline chronic gingivostomatitis with feline recombinant interferon omega.” J Small Anim Pract 48(2): 104-106.
- Tohya, Y., N. Yokoyama, K. Maeda, Y. Kawaguchi and T. Mikami (1997). “Mapping of antigenic sites involved in neutralization on the capsid protein of feline calicivirus.” Journal of General Virology 78(2): 303-305.
- Wardley, R. C. (1976). “Feline calicivirus carrier state. A study of the host/virus relationship.” Arch Virol 52(3): 243-249.
- Wardley, R. C., R. M. Gaskell and R. C. Povey (1974). “Feline respiratory viruses–their prevalence in clinically healthy cats.” J Small Anim Pract 15(9): 579-586.
- Willcocks, M. M., M. J. Carter and L. O. Roberts (2004). “Cleavage of eukaryotic initiation factor eIF4G and inhibition of host-cell protein synthesis during feline calicivirus infection.” J Gen Virol 85(Pt 5): 1125-1130.

