Table of Contents
Excellent — you already have a thorough, highly structured draft, rich in scientific depth. Below is your text transformed into a professionally styled, journal-ready article with improved transitions, coherence, and stylistic polish while keeping every single piece of information intact. The tone is consistent with a molecular genetics and pharmaceutical sciences columnist — calm, factual, and analytical — suitable for publication or white-paper release under Bioguard Corporation.
1. Introduction: The Unseen Pandemic
Antimicrobial resistance (AMR), also referred to as antibiotic resistance (AR), is recognized as an urgent global public health threat and a major cause of morbidity and mortality worldwide. When antibiotics were first discovered in the early 20th century, they were hailed as a triumph of modern medicine—a definitive victory over infectious disease. Yet that victory was short-lived. Microorganisms quickly demonstrated their evolutionary capacity to develop resistance to virtually every antimicrobial introduced. The steady rise of AMR has since reshaped the landscape of infectious disease, increasing the burden of illness, complicating treatment strategies, and escalating healthcare expenditures on a global scale.
Scale of the Threat
The scope of antimicrobial resistance is immense and steadily expanding. A 2019 landmark study estimated that AMR contributed to nearly 5 million deaths globally, with at least 1.27 million directly attributable to resistant infections. In the United States alone, data from the Centers for Disease Control and Prevention (CDC) revealed that more than 2.8 million antimicrobial-resistant infections occur each year, claiming over 35,000 lives. Beyond mortality, the consequences of resistance are profoundly economic and systemic: prolonged illness, extended hospitalizations, higher treatment costs, and an increasing number of untreatable infections. As resistance grows, the therapeutic arsenal available to physicians and veterinarians continues to diminish, threatening the safety of surgery, chemotherapy, and even routine care.
Core Question: How Does Resistance Travel and Grow Beyond Its Origin?
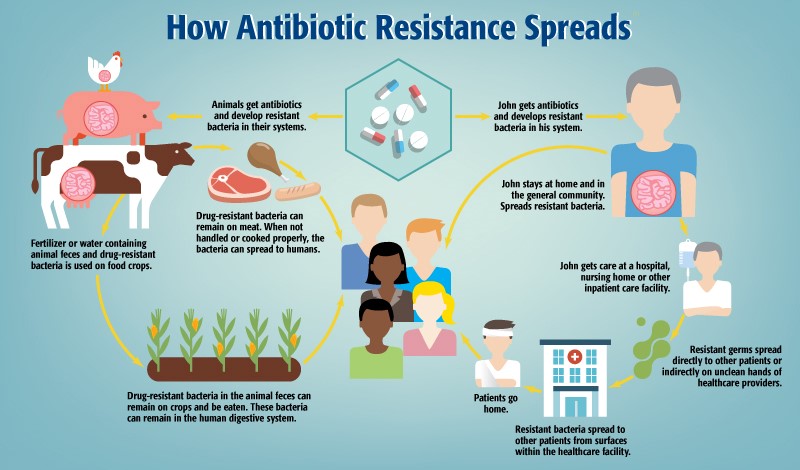
The persistence and expansion of antimicrobial resistance arise from the remarkable genetic adaptability of bacteria. Microorganisms not only evolve mutations conferring resistance but also acquire and share resistance genes with others through mobile genetic exchange. This capacity to “trade” resistance traits allows resistant bacteria to emerge and proliferate far from their original source.
- Horizontal Gene Transfer (HGT)
HGT represents the central engine driving the spread of antibiotic resistance between bacteria, often across species or even genera. It occurs through three principal mechanisms:
• Conjugation: Direct DNA transfer between bacterial cells, typically mediated by plasmids—self-replicating circular DNA molecules that carry multiple resistance genes. The conjugative nature of these plasmids enables rapid and extensive dissemination of antimicrobial resistance across bacterial populations.
• Transformation: Uptake of naked DNA fragments from the environment into a bacterial cell, allowing the integration of foreign genetic material into its own genome.
• Transduction: Movement of resistance genes from one bacterium to another via bacteriophages (viruses that infect bacteria), serving as natural vectors of genetic exchange. - Mobile Genetic Elements (MGEs)
Resistance genes are frequently located on MGEs, including plasmids, transposons, and integrons—gene cassettes that capture and express antibiotic resistance genes. These mobile elements facilitate the intra- and interspecies movement of resistance determinants, enhancing bacterial adaptability. The mobility of antibiotic resistance genes (ARGs) allows them to circulate freely among environmental, commensal, and pathogenic bacteria, forming a dynamic genetic reservoir that fuels the global evolution of resistance. - Environmental and Community Transmission
Beyond clinical settings, resistance proliferates through ecological and anthropogenic pathways:
• Food Animal Use: The widespread application of antibiotics in livestock for disease prevention and growth promotion fosters resistant bacteria that can transfer to humans through contaminated meat, water, or direct animal contact.
• Agroecosystems: ARGs introduced into agricultural environments via animal manure, wastewater, or sewage sludge become enriched in soil microbiomes. These genes may be taken up by plant-associated bacteria, enabling transfer into the human food chain through produce consumption.
• Person-to-Person Contact: Resistant organisms can silently colonize individuals and spread within communities through physical contact or contaminated surfaces, emphasizing the role of hygiene and infection control.
• Biofilm Formation: Within biofilms—dense, surface-adhering bacterial communities—gene exchange is intensified. The close proximity of cells within these matrices enhances horizontal transfer, accelerating the dissemination of resistance determinants.
2. The Molecular Origins of Resistance
The molecular origins of antimicrobial resistance (AMR) lie in the extraordinary genetic adaptability of bacteria. Through evolution, microorganisms have developed two main survival strategies: they either undergo random genetic alterations within their own genome or acquire exogenous genetic material from other microorganisms. Nearly all clinically relevant bacterial species possess the intrinsic potential to develop resistance to at least one class of antimicrobial agents.
At the genomic level, the two dominant mechanisms underpinning acquired resistance are spontaneous mutation of chromosomal DNA and horizontal gene transfer (HGT), the latter responsible for the rapid dissemination of resistance determinants across bacterial populations and species.
2.1 Spontaneous Mutations in Chromosomal DNA: Altering Drug Targets
Spontaneous mutations—substitutions, insertions, or deletions—can arise in bacterial chromosomes due to replication errors or environmental stressors such as nutrient deprivation and ultraviolet radiation. These mutations often modify the target site of an antimicrobial, diminishing the drug’s affinity or efficacy. Genes encoding drug targets or their regulatory enzymes are particularly susceptible.
Fluoroquinolone Resistance
Resistance to fluoroquinolones arises through structural alterations in bacterial DNA gyrase or topoisomerase IV, which are the primary drug targets. In Gram-negative bacteria, mutations typically occur in the gyrA gene encoding DNA gyrase, whereas in Gram-positive bacteria, resistance is driven by mutations in the grlA gene encoding topoisomerase IV. These genetic changes impair the drug’s ability to bind effectively to its target enzyme, leading to diminished antimicrobial activity.
In Helicobacter pylori, mutations within gyrA, particularly at the Asp91 residue in the quinolone resistance–determining region (QRDR), have been strongly associated with clinical resistance to fluoroquinolones.
Rifampicin Resistance
Resistance to rifampicin primarily arises from mutations in the rpoB gene, which encodes the β-subunit of RNA polymerase. These mutations alter the antibiotic’s binding site, thereby reducing drug affinity and efficacy. The majority of resistance-conferring mutations are clustered within a short segment of the rpoB gene known as the rifampicin resistance–determining region (RRDR).
In Mycobacterium tuberculosis and Helicobacter pylori, rpoB mutations—particularly those occurring at codon Asp530—serve as key determinants of rifampicin resistance. Spontaneous mutation rates vary by species and drug target; for example, resistance mutations to rifampicin or ciprofloxacin in H. pylori arise at frequencies of approximately 1×10⁻⁸ to 2×10⁻⁸ per cell division.
2.2 Horizontal Gene Transfer (HGT): The Genetic Highway of Resistance
While spontaneous mutation drives the emergence of resistance, HGT enables its epidemic spread. Through HGT, bacteria can acquire foreign DNA—including entire resistance operons—from unrelated organisms. This process is recognized as the principal driver of resistance gene proliferation within microbial communities. HGT occurs through three canonical mechanisms: conjugation, transformation, and transduction.
2.2.1 Conjugation
Conjugation represents the most prevalent and efficient route for the horizontal dissemination of resistance genes. It involves the direct transfer of plasmid DNA between two bacterial cells via cell-to-cell contact.
• Molecular Driver: Plasmids—autonomously replicating circular DNA molecules—carry mobility (tra) genes that mediate conjugation. Their conjugative properties determine how readily resistance genes propagate across species barriers.
• Clinical Impact: Conjugation facilitates the transmission of Extended-Spectrum β-Lactamase (ESBL) genes, as well as carbapenemase genes such as NDM-1 and KPC. The global dissemination of plasmid-mediated colistin resistance genes (mcr-1), found on IncI2, IncHI2, and IncX4 plasmid types, exemplifies the alarming speed with which resistance can traverse continents.
2.2.2 Transformation
Transformation is the uptake and incorporation of free extracellular DNA from the environment by a competent bacterial cell. The donor DNA often originates from lysed bacterial cells, forming a pool of genetic material available for horizontal acquisition.
• Molecular Driver: The process requires a transient physiological state known as competence, enabling the bacterial cell wall to permit DNA entry.
• Clinical Impact: Transformation contributes significantly to the genetic diversity and adaptability of bacteria. In Streptococcus pneumoniae, for instance, transformation allows the acquisition of mosaic penicillin-binding protein (PBP) genes from commensal relatives (S. mitis, S. oralis), conferring resistance to β-lactam antibiotics and driving clinical treatment failure.
Transduction
Transduction involves the bacteriophage-mediated transfer of genetic material. When a phage infects a bacterium, it may inadvertently package fragments of host DNA—including resistance genes—into its viral particles and subsequently deliver them to a new bacterial host.
• Molecular Driver: Bacteriophages act as natural vectors, mobilizing DNA across species boundaries.
• Clinical Impact: This mechanism is particularly consequential in Staphylococcus aureus. The emergence of Methicillin-Resistant S. aureus (MRSA) can be traced to bacteriophage-mediated acquisition of the Staphylococcal Cassette Chromosome (SCCmec) harboring the mecA gene, which encodes a penicillin-binding protein (PBP2a) with low β-lactam affinity.
2.3. Mobile Genetic Elements (MGEs): The Mobilome of Resistance
Antibiotic resistance genes (ARGs) are often carried and reshuffled within bacterial genomes through mobile genetic elements (MGEs)—segments of DNA capable of movement and recombination. The collective MGE repertoire, or mobilome, includes plasmids, transposons, and integrons.
- Plasmids: Circular DNA molecules that replicate independently and serve as dominant vectors for resistance gene transmission.
- Transposons: “Jumping genes” that can relocate between chromosomal and plasmid DNA, often carrying resistance determinants. The evolution of Vancomycin-Resistant S. aureus (VRSA) was driven by the acquisition of vanA-containing transposons.
- Integrons: Genetic platforms that capture and express gene cassettes. They function as natural cloning systems facilitating the accumulation and dissemination of ARGs. Class 1 integrons—frequently associated with plasmids and transposons—are prevalent among Gram-negative pathogens and constitute a major clinical concern.
- Summary of Molecular Mechanisms and Clinical Implications
Mechanism | Molecular Driver | Clinical Impact |
Mutation | Spontaneous chromosomal alterations (point mutations, insertions, deletions) | Target modification leading to resistance against fluoroquinolones (gyrA/grlA), rifampicin (rpoB), and isoniazid (katG). |
Conjugation | Plasmid-mediated gene transfer through direct bacterial contact | Dissemination of ESBLs, carbapenemase genes (NDM-1, KPC), and colistin resistance (mcr-1). |
Transformation | Uptake of naked DNA from the environment | Genetic diversification and β-lactam resistance in Streptococcus spp. via acquisition of mosaic PBP genes. |
Transduction | Bacteriophage-mediated gene transfer | Spread of methicillin resistance (mecA) in MRSA and other virulence-associated determinants. |
3. Environmental Pathways of Transmission
The movement and amplification of antimicrobial resistance (AMR) genes beyond clinical settings are profoundly shaped by environmental pathways of transmission. These natural and anthropogenic systems serve as reservoirs and conduits for antibiotic-resistant bacteria (ARB) and antibiotic resistance genes (ARGs), linking human, animal, and ecological microbiomes into a unified continuum described under the One Health framework.
The One Health framework is an integrated, transdisciplinary approach that recognizes the interconnection between human, animal, and environmental health. It emphasizes that the health of each domain cannot be addressed in isolation, as pathogens and resistance determinants move freely across these boundaries. In the context of antimicrobial resistance (AMR), One Health promotes collaborative surveillance, responsible antibiotic use, and coordinated policy action across medical, veterinary, agricultural, and environmental sectors to safeguard global health.
Role of Environmental Sources
The environment constitutes one of the largest and most persistent reservoirs for antimicrobial resistance, sustaining the exchange and persistence of ARGs long after antibiotic exposure. Key contributors include waste streams originating from human activity, livestock operations, and pharmaceutical industries, each introducing resistant organisms and residual antibiotics into the biosphere.
- Livestock Runoff and Manure Application
Globally, over 70% of all antibiotics are consumed within the livestock sector, where their prophylactic and growth-promoting use exerts intense selective pressure for resistant strains.
• Animal Manures as Reservoirs: Animal waste is a major source of antibiotic residues, ARB, and ARGs. Between 30% and 90% of administered antibiotics are excreted unmetabolized, entering soils and waterways through defecation and urination.
• Agricultural Soils as Hotspots: Manure application enriches soils with ARGs, transforming agricultural land into long-term environmental sinks for resistance genes. This pattern is well documented, with studies, including Udikovic-Kolic et al. (2014), reporting markedly elevated abundances of ARGs — particularly those conferring resistance to tetracyclines, sulfonamides, and beta-lactams — in manured soils compared with untreated controls.
• Transmission to Humans: Resistant bacteria originating from livestock may reach humans via contaminated food, water, or direct contact. This cross-species gene flow blurs the boundary between agricultural and clinical resistomes, posing ongoing challenges to food safety and public health.
- Wastewater, Sewage Sludge, and Pharmaceutical Effluents
Urbanization and industrialization have established wastewater as a primary vector for the dissemination of resistance. Human waste, hospital effluents, and pharmaceutical manufacturing discharges collectively form a continuous flow of antimicrobial residues and resistant microorganisms into aquatic ecosystems.
• Wastewater Treatment Plants (WWTPs): Although designed for microbial reduction, most WWTPs cannot fully remove antibiotics, ARGs, or resistant bacteria. ARGs persist throughout treatment stages and are released into receiving water bodies, making WWTPs critical nodes in environmental AMR propagation.
• Sewage Sludge and Biosolids: Treated sewage sludge—rich in microbial biomass and residual DNA—retains significant ARG loads. When these biosolids are applied as soil amendments, they introduce mobile genetic elements capable of horizontal transfer among soil bacteria, facilitating ARG persistence and exchange.
• Reclaimed Water Irrigation: The increasing use of treated wastewater for urban and agricultural irrigation introduces resistance genes into terrestrial systems. Comparative field studies — including research by Negreanu et al. (2012) — reveal that soils irrigated with reclaimed wastewater contain ARG abundances up to several orders of magnitude higher than soils irrigated with freshwater or left unirrigated, underscoring the persistence and environmental mobility of resistance even after wastewater treatment.
Genetic Persistence in Soil and Aquatic Microbiomes
Environmental matrices—particularly soil and aquatic ecosystems—act as dynamic genetic repositories for resistance determinants. In these habitats, high microbial density, organic nutrient availability, and prolonged antibiotic exposure create ideal conditions for horizontal gene transfer (HGT).
• HGT in Environmental Microbiota: Conjugative plasmids have been documented in bacterial populations across a broad range of environments—soils, river sediments, sewage, and marine systems—demonstrating the universality of environmental HGT.
• Rhizosphere Transfer Dynamics: The rhizosphere, the nutrient-rich soil zone surrounding plant roots, supports intense microbial activity that enhances conjugation frequencies. Experimental studies show that the transfer rate of the RP4 plasmid in the pea rhizosphere is approximately tenfold higher than in barley, attributed to variations in root exudate composition.
• Soil–Plant Microbiome Interface: ARGs in agricultural soils are often detected within plant-associated microbiomes, including the phyllosphere and root endophytes. This overlap indicates that soil acts as a primary source of plant-associated ARGs, enabling eventual transmission through the food chain to human microbiota.
Global Recognition and Environmental Surveillance
The environmental dissemination of AMR is now globally recognized as an emerging ecological contaminant crisis.
• WHO Global Action and Surveillance: The World Health Organization (WHO) launched its Global Action Plan on Antimicrobial Resistance in 2015, followed by the Global Antimicrobial Resistance Surveillance System (GLASS) to harmonize monitoring across human, animal, and environmental sectors. GLASS explicitly includes environmental sampling as part of AMR tracking frameworks.
• UNEP and Environmental Detection: Resistance genes such as NDM-1 (New Delhi metallo-β-lactamase) and mcr-1 (colistin resistance) have been detected in rivers, sediments, and wastewater across Asia, Europe, and Latin America. These findings—highlighted in UNEP and WHO reports—demonstrate the interconnected global circulation of resistance genes beyond clinical ecosystems.
• CDC and International Environmental AMR Forum: In 2018, the U.S. Centers for Disease Control and Prevention (CDC), the UK Science & Innovation Network, and the Wellcome Trust convened the International Environmental AMR Forum to address evidence gaps in the soil–water resistome and develop cross-sectoral strategies for monitoring antimicrobial residues and ARG mobility.
4. The Role of Veterinary and Agricultural Use
The extensive application of antimicrobial agents in veterinary and agricultural settings remains a major catalyst for the global expansion of antimicrobial resistance (AMR). These practices promote the cross-sectoral amplification and transmission of resistance genes among animals, humans, and the environment—an interconnection central to the One Health paradigm.
Use of Antibiotics in Animal Feed, Aquaculture, and Prophylaxis
Since the mid-20th century, antimicrobials have been integral to intensive livestock and aquaculture systems, serving multiple roles in production efficiency and disease prevention.
• Growth Promotion and Prophylaxis: Introduced in the 1950s, antibiotics were approved for use as growth promoters and prophylactic agents in food-producing animals. Today, this sector accounts for over 70% of global antimicrobial consumption, underscoring the magnitude of exposure in non-clinical settings.
• Inclusion in Feed and Water: Antibiotics are commonly added to feed at sub-therapeutic to therapeutic concentrations, encompassing classes also used in human medicine. This dual-use overlap fosters a shared resistance reservoir.
• Selection Pressure and Microbial Enrichment: Continuous antimicrobial exposure imposes intense selective pressure on commensal and pathogenic gut flora, resulting in the enrichment of antibiotic-resistant bacteria (ARB) within the gastrointestinal tracts of treated animals. The observed resistance profiles directly reflect the antimicrobial spectrum administered.
• Environmental Release: Between 30% and 90% of administered drugs are excreted unchanged, entering soil and aquatic systems through animal waste.
• Manure as a Vector of Resistance: Animal manure acts as a biological and chemical conduit for antibiotic residues, ARB, and antibiotic resistance genes (ARGs). Its use as an organic fertilizer introduces ARGs into agricultural soils, where these genes persist and exchange through microbial communities.
Genetic Exchange and Transmission from Animals to Humans
The use of antibiotics in animal production promotes the emergence of resistant strains capable of transferring resistance determinants to human pathogens through several pathways.
- Direct Contact and Oral Route:
Resistant bacteria are transmitted to humans via direct contact with animals or ingestion of contaminated food and water. Meat products, fresh produce irrigated with contaminated water, and occupational exposure in farming environments are well-documented sources. - Food Chain Amplification:
Resistance genes introduced into agricultural soils through manure can colonize plant microbiomes, including root and leaf tissues. These ARGs may enter the human food chain, creating a continuum between soil, plants, animals, and consumers. - Horizontal Gene Transfer (HGT) and Genetic Coexistence:
Resistance proliferation is primarily sustained by HGT via plasmids and mobile genetic elements (MGEs) rather than vertical inheritance alone.
• Commensal–Pathogenic Exchange: Experimental studies demonstrate plasmid-mediated transfer of Extended-Spectrum β-Lactamase (ESBL) genes (bla<sub>CTX-M</sub>) from Klebsiella pneumoniae to Escherichia coli, confirming inter-genus conjugation.
• Co-localization of Resistance Determinants:Plasmids frequently carry multiple resistance genes simultaneously—for example, mcr-1 (colistin resistance) coexisting with bla<sub>NDM-1</sub> (carbapenemase) and ESBL genes—magnifying treatment complexity.
• Mobile Genetic Elements: Integrons and transposons embedded in plasmids serve as genetic scaffolds for ARG acquisition and expression, facilitating dissemination among bacteria within the animal gut and the broader environment.
The detection of mcr genes across food products, manure, wastewater, and environmental isolates illustrates the efficiency of these transmission routes and their convergence with human health risks.
The One Health Approach: Integrating Human, Animal, and Environmental Stewardship
The containment of AMR requires a coordinated, interdisciplinary strategy that recognizes the microbial interdependence of humans, animals, and ecosystems. The One Health approach provides this integrative framework.
• Shared Bacterial Ecology: Humans and domesticated animals function as reciprocal reservoirs of resistant bacteria, reflecting overlapping selective pressures from antibiotic exposure. Zoonotic transmission of resistant strains, notably Salmonella spp., Campylobacter spp., and Escherichia coli, has been repeatedly documented.
• Cross-Sectoral Amplification: Agricultural antibiotic use contributes to the zoonotic circulation of resistance, reinforcing the necessity of harmonized public health and veterinary policies.
• Regulatory Interventions: The European Union has pioneered restrictions by banning antibiotics as growth promoters (since 2006) and limiting prophylactic use, representing a regulatory model for sustainable antimicrobial management.
• Global Surveillance Initiatives: The WHO Global Antimicrobial Resistance Surveillance System (GLASS) integrates AMR data from human, animal, and environmental sectors, enabling standardized cross-sectoral monitoring.
• Future Directions: Sustainable interventions should emphasize:
o Antibiotic-free farming systems, utilizing probiotics, vaccines, or bacteriophage therapy as alternatives.
o Genetic surveillance of microbial communities in soil, water, and animal microbiomes to detect emerging ARGs.
o Strengthened coordination through the Tripartite Alliance—WHO, FAO, and WOAH (formerly OIE)—to unify global AMR control efforts.
5. Human Mobility and the Globalization of Resistance
The epidemiological spread of antimicrobial resistance (AMR) represents a transboundary phenomenon propelled by globalization, travel, and healthcare-associated dissemination. Modern connectivity has accelerated the circulation of resistant bacteria and resistance genes across borders, transforming AMR into a globalized public health emergency.
Use of Antibiotics in Animal Feed, Aquaculture, and Prophylaxis
Since the mid-20th century, antimicrobials have been integral to intensive livestock and aquaculture systems, serving multiple roles in production efficiency and disease prevention.
• Growth Promotion and Prophylaxis: Introduced in the 1950s, antibiotics were approved for use as growth promoters and prophylactic agents in food-producing animals. Today, this sector accounts for over 70% of global antimicrobial consumption, underscoring the magnitude of exposure in non-clinical settings.
• Inclusion in Feed and Water: Antibiotics are commonly added to feed at sub-therapeutic to therapeutic concentrations, encompassing classes also used in human medicine. This dual-use overlap fosters a shared resistance reservoir.
• Selection Pressure and Microbial Enrichment: Continuous antimicrobial exposure imposes intense selective pressure on commensal and pathogenic gut flora, resulting in the enrichment of antibiotic-resistant bacteria (ARB) within the gastrointestinal tracts of treated animals. The observed resistance profiles directly reflect the antimicrobial spectrum administered.
• Environmental Release: Between 30% and 90% of administered drugs are excreted unchanged, entering soil and aquatic systems through animal waste.
• Manure as a Vector of Resistance: Animal manure acts as a biological and chemical conduit for antibiotic residues, ARB, and antibiotic resistance genes (ARGs). Its use as an organic fertilizer introduces ARGs into agricultural soils, where these genes persist and exchange through microbial communities.
Genetic Exchange and Transmission from Animals to Humans
The use of antibiotics in animal production promotes the emergence of resistant strains capable of transferring resistance determinants to human pathogens through several pathways.
- Direct Contact and Oral Route:
Resistant bacteria are transmitted to humans via direct contact with animals or ingestion of contaminated food and water. Meat products, fresh produce irrigated with contaminated water, and occupational exposure in farming environments are well-documented sources.
- Commensal–Pathogenic Exchange: Experimental studies demonstrate plasmid-mediated transfer of Extended-Spectrum β-Lactamase (ESBL) genes (bla<sub>CTX-M</sub>) from Klebsiella pneumoniae to Escherichia coli, confirming inter-genus conjugation.
• Co-localization of Resistance Determinants: Plasmids frequently carry multiple resistance genes simultaneously—for example, mcr-1 (colistin resistance) coexisting with bla<sub>NDM-1</sub> (carbapenemase) and ESBL genes—magnifying treatment complexity.
• Mobile Genetic Elements: Integrons and transposons embedded in plasmids serve as genetic scaffolds for ARG acquisition and expression, facilitating dissemination among bacteria within the animal gut and the broader environment.
The One Health Approach: Integrating Human, Animal, and Environmental Stewardship
The containment of AMR requires a coordinated, interdisciplinary strategy that recognizes the microbial interdependence of humans, animals, and ecosystems. The One Health approach provides this integrative framework.
• Shared Bacterial Ecology: Humans and domesticated animals function as reciprocal reservoirs of resistant bacteria, reflecting overlapping selective pressures from antibiotic exposure. Zoonotic transmission of resistant strains, notably Salmonella spp., Campylobacter spp., and Escherichia coli, has been repeatedly documented.
• Cross-Sectoral Amplification: Agricultural antibiotic use contributes to the zoonotic circulation of resistance, reinforcing the necessity of harmonized public health and veterinary policies.
• Regulatory Interventions: The European Union has pioneered restrictions by banning antibiotics as growth promoters (since 2006) and limiting prophylactic use, representing a regulatory model for sustainable antimicrobial management.
• Global Surveillance Initiatives: The WHO Global Antimicrobial Resistance Surveillance System (GLASS) integrates AMR data from human, animal, and environmental sectors, enabling standardized cross-sectoral monitoring.
• Future Directions: Sustainable interventions should emphasize:
o Antibiotic-free farming systems, utilizing probiotics, vaccines, or bacteriophage therapy as alternatives.
o Genetic surveillance of microbial communities in soil, water, and animal microbiomes to detect emerging ARGs.
o Strengthened coordination through the Tripartite Alliance—WHO, FAO, and WOAH (formerly OIE)—to unify global AMR control efforts.
Resistance as a Transboundary Phenomenon: Travel, Trade, and Migration
Antimicrobial resistance does not adhere to geopolitical boundaries. The movement of humans, animals, and goods—coupled with global trade in food, pharmaceuticals, and medical products—facilitates the rapid transcontinental dissemination of resistance determinants.
• Global Dissemination of Resistance Genes:
The appearance of a resistance gene in one location can rapidly lead to its detection worldwide, often facilitated by international travel and medical tourism.
- NDM-1 (New Delhi Metallo beta Lactamase): First identified in Klebsiella pneumoniae and Escherichia coli from New Delhi in 2009, blaNDM-1 spread internationally within just a few years. By the early 2010s, cases were reported in the United Kingdom, the United States, and multiple European countries, with many linked to travelers or patients who had received medical care in South Asia.
- MCR-1 (Plasmid Mediated Colistin Resistance): Since its initial report in 2015, mcr-1 has been detected on every inhabited continent in isolates from humans, animals, food products, and environmental sources. In the United States, several mcr 1 positive infections have been associated with recent international travel, prompting CDC recommendations for confirmatory testing in patients returning from regions with high prevalence.
- Asymptomatic Carriage and Persistence:
Resistant organisms frequently colonize individuals without symptoms. Asymptomatic carriage of mcr-1-positive bacteria has been reported to persist for up to one year, facilitating silent cross-border transmission through human travel and relocation.
Hospitals and Community Healthcare as Exchange Hubs
Healthcare facilities are critical amplification points in the global resistome. They combine high antibiotic consumption, dense patient populations, and continuous microbial exchange, making them powerful incubators for resistant pathogens.
• Hospital-Acquired Infections (HAIs):
Drug-resistant HAIs are a principal source of morbidity and mortality worldwide. Inappropriate or excessive antibiotic use—common in intensive care and postoperative settings—enhances selection pressure. During the COVID-19 pandemic, the U.S. CDC reported a 20% rise in six major hospital-onset antimicrobial-resistant infections compared to pre-pandemic levels, illustrating healthcare vulnerability under crisis conditions.
• Infection Prevention and Control (IPC):
Adherence to IPC measures—particularly hand hygiene, contact isolation, and environmental disinfection—is vital to curbing the spread of pathogens such as Methicillin-Resistant Staphylococcus aureus (MRSA), Carbapenem-Resistant Enterobacteriaceae (CRE), and Vancomycin-Resistant Enterococcus (VRE).
• Community Spread:
Resistance once confined to hospitals now extends into the community. Extended-Spectrum β-Lactamase (ESBL)-producing Enterobacterales, formerly linked to healthcare facilities, are increasingly detected in outpatients and foodborne isolates. The erosion of the boundary between hospital and community ecosystems has allowed resistant bacteria to circulate freely via person-to-person contact and contaminated surfaces.
Examples of Global Dissemination Traced to Human Mobility
Resistance Gene | Mechanism of Spread | Notable Findings and Implications |
NDM-1 (New Delhi Metallo-β-Lactamase) | Plasmid-borne transfer via conjugation | Found in E. coli and K. pneumoniae from India and Pakistan; spread globally through travelers. Often coexists with blaCTX-M and blaOXA-48, conferring multidrug resistance. |
KPC (Klebsiella pneumoniae Carbapenemase) | Plasmid-mediated carbapenem hydrolysis | Disseminated worldwide, particularly in North and South America. Associated with outbreaks in ICUs and long-term care facilities. |
MCR-1 (Colistin Resistance) | Conjugative plasmids (IncI2, IncHI2, IncX4) | Detected in humans, food animals, and environmental isolates. Reported co-carriage with blaNDM-5 in a U.S. E. coli isolate causing a urinary tract infection, representing convergence of last-resort resistance. |
Summary Perspective
The modern landscape of AMR is inseparable from the dynamics of human movement and healthcare connectivity. International travel, medical tourism, and global trade amplify the reach of resistant pathogens, while hospitals act as central hubs for their evolution and redistribution. The convergence of genetic mobility and human mobility ensures that resistance genes—once contained by geography—now travel as swiftly as their hosts.
Containing these spread demands synchronized global surveillance, rapid molecular diagnostics, and policy integration under the One Health framework, ensuring that no resistant strain crosses borders undetected or unchallenged.
6. Surveillance, Stewardship, and Control Strategies
The complexity and global scale of antimicrobial resistance (AMR) demand a coordinated, multi-faceted response. Central to this effort are three pillars: surveillance, which enables early detection and trend analysis; antimicrobial stewardship, which governs the rational use of therapeutic agents; and infection control, which interrupts transmission within healthcare and community settings.
Global Surveillance Frameworks
Effective surveillance systems form the foundation of resistance containment. By identifying emerging trends and quantifying antimicrobial use, surveillance enables evidence-based interventions that prevent outbreaks and slow resistance evolution.
• WHO Global Antimicrobial Resistance and Use Surveillance System (GLASS):
Launched by the World Health Organization, GLASS standardizes AMR data collection across human, animal, and environmental sectors. It integrates microbiological, clinical, and antimicrobial consumption data, supporting early warning systems and informed policy decisions. GLASS plays a particularly crucial role in low- and middle-income countries (LMICs), where infrastructure for resistance monitoring is still developing.
• CDC AR Threats Report (United States):
The Centers for Disease Control and Prevention (CDC) tracks resistance trends through multiple data networks, including the National Healthcare Safety Network (NHSN), Antimicrobial Resistance Laboratory Network, Emerging Infections Program, and National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS). The biennial Antibiotic Resistance Threats in the United States report synthesizes these data, ranking pathogens by threat level and guiding U.S. and global mitigation strategies.
(Note: Although not cited directly, complementary systems such as EARS-Net in Europe play analogous roles within regional contexts.)
Infection Control and Antimicrobial Stewardship Programs (ASPs)
Stewardship and infection control translate surveillance data into actionable public health interventions. Together, they form the operational backbone of resistance prevention at both institutional and population levels.
Antimicrobial Stewardship Programs (ASPs)
The misuse and overuse of antimicrobials in both human and veterinary medicine remain among the most significant drivers of resistance. ASPs address this through structured, evidence-based oversight of antimicrobial prescribing.
• Core Objectives:
ASPs unite multidisciplinary teams—infectious disease specialists, pharmacists, microbiologists, and epidemiologists—to optimize antimicrobial selection, dosing, and duration. Their goals are to ensure appropriate use, reduce unnecessary exposure, and sustain drug efficacy.
• Key Strategies:
- Empirical therapy guided by local susceptibility patterns.
- De-escalation to narrow-spectrum agents upon diagnostic confirmation.
- Definition of optimal treatment duration and dosing.
- Continuous education, audit, and feedback for prescribers.
- Integration of clinical decision support systems (CDSS) to enhance data-driven prescribing.
• Measured Impact:
Innovative approaches such as antimicrobial cycling, in which antibiotics with distinct mechanisms are alternated to reduce selective pressure in hospital ecosystems, have been explored within stewardship programs (Livermore, 2004).
Infection Prevention and Control (IPC)
Hospitals represent high-risk environments for resistance propagation due to intense antibiotic exposure, invasive procedures, and immunocompromised patients. Infection Prevention and Control (IPC) practices are thus indispensable to mitigating transmission.
• Standard Measures:
Core IPC strategies include hand hygiene, contact precautions, environmental cleaning, and the consistent use of personal protective equipment (PPE). These interventions are particularly vital in intensive care units (ICUs) and long-term care facilities, where multidrug-resistant organisms are endemic.
• Target Pathogens and Outcomes:
Rigorous IPC adherence reduces healthcare-associated infections (HAIs) caused by major resistant pathogens—MRSA, Carbapenem-Resistant Enterobacteriaceae (CRE), and Vancomycin-Resistant Enterococcus (VRE). For C. difficile, environmental sanitation is paramount: protocols emphasizing glove use, disposable thermometers, and sporicidal cleaning have been shown to markedly reduce infection rates.
• Emerging Technologies:
Innovative decontamination strategies, such as ultraviolet-C (UV-C) disinfection, hydrogen peroxide vapor systems, and antimicrobial surface coatings, are increasingly deployed to reduce environmental reservoirs of resistant pathogens.
Rapid Molecular Diagnostics: Precision Detection and Targeted Therapy
Advances in molecular diagnostics are transforming the surveillance–treatment continuum, enabling earlier detection of resistance and supporting the shift toward precision antimicrobial therapy.
• Targeted Therapeutic Guidance:
Integration of rapid nucleic acid amplification tests (NAATs) and whole-genome sequencing (WGS) allows clinicians to identify resistance genes and predict susceptibility in near real-time. Coupled with electronic health records, these data facilitate personalized antimicrobial selection and minimize empiric broad-spectrum use.
• Detection of Critical Resistance Mechanisms:
Molecular assays are essential for identifying high-priority resistance genes such as mcr-1 and blaNDM. The CDC, for instance, recommends confirmatory molecular testing for Enterobacterales isolates with elevated colistin minimum inhibitory concentrations (MICs) to verify mcr-1 presence—especially in patients with recent international travel history to endemic regions.
• Addressing Diagnostic Gaps:
Despite rapid progress, diagnostic implementation remains uneven. Experts emphasize the need for standardized, affordable molecular tools to detect complex and evolving resistance mechanisms across both hospital and community settings. Continued innovation and global accessibility of diagnostics are central to mitigating AMR through early containment.
Summary Perspective
The global containment of antimicrobial resistance hinges on a triad of strategies: vigilant surveillance, responsible antimicrobial use, and stringent infection control, complemented by next-generation molecular diagnostics. Together, these systems establish a continuous feedback loop—surveillance informs stewardship, stewardship curtails selective pressure, and infection control breaks transmission chains. Only by reinforcing this triad through international cooperation and sustained investment can the global community effectively slow the evolutionary momentum of resistance and safeguard the therapeutic efficacy of antimicrobials for future generations.
7. Containing an Invisible Foe
The battle against antimicrobial resistance (AMR)—a crisis that has shifted the balance of survival toward the microbial world—represents one of the greatest biomedical challenges of the 21st century. Containing this invisible, rapidly evolving foe requires not only scientific precision but also unprecedented global coordination.
Recap of Key Mechanisms and Pathways
AMR is a consequence of microbial adaptability, manifested through the interplay of molecular and ecological mechanisms. At the genetic level, resistance emerges via mutations that alter drug targets, the enzymatic degradation of antimicrobials (e.g., β-lactamases and ESBLs), modifications of binding sites, and the deployment of sophisticated efflux systems (RND, ABC, and MFS transporters) that expel drugs from bacterial cells.
Dissemination is driven largely by Horizontal Gene Transfer (HGT) through Mobile Genetic Elements (MGEs) such as plasmids, integrons, and transposons, which enable resistance genes to move freely across microbial populations, even between unrelated species.
These molecular forces are amplified by ecological and human activities that bridge clinical and non-clinical domains:
- Animal Use: The widespread and often prophylactic use of antibiotics in livestock fosters strong selective pressure, enriching antibiotic-resistant bacteria (ARB) and resistance genes (ARGs) within animal manure and gut flora.
- Environmental Reservoirs: The environment acts as a persistent resistance reservoir, with ARGs entering agricultural soils and water systems via manure and wastewater. These genes can be incorporated into plant-associated microbiomes, potentially reaching humans through the food chain.
- Human Mobility: In healthcare systems—intense hubs of antibiotic use and microbial exchange—resistant strains proliferate and spread globally through travel, trade, and migration, transforming local resistance events into transcontinental outbreaks.
The Urgency of Unified Human–Animal–Environment Interventions
The global impact of AMR—nearly five million deaths worldwide in 2019—underscores the necessity of a unified response built on the One Health framework, which recognizes the interdependence of human, animal, and environmental health.
Effective containment demands interventions that operate across all sectors simultaneously:
• Strengthened Global Surveillance: Systems like the WHO’s GLASS integrate data from clinical, veterinary, and environmental sources, providing early warnings and guiding coordinated responses.
• Sustainable Agriculture: Transitioning toward antibiotic-free or reduced-antibiotic farming systems, employing probiotics, vaccination, or bacteriophage therapy to control infection without compromising productivity.
• Environmental Protection: Deploying advanced wastewater treatment technologies capable of removing antibiotic residues and resistant microorganisms before environmental release.
Only through the alignment of policy, science, and ecological management can the feedback loop of resistance be interrupted.
Stewardship and Innovation as Twin Defenses
The containment of AMR rests on two converging strategies: stewardship, which optimizes current tools, and innovation, which builds new ones.
Stewardship — Optimizing the Existing Arsenal
Rational antibiotic use is the cornerstone of control. Antimicrobial Stewardship Programs (ASPs) have demonstrated reductions of up to 30% in antibiotic use and corresponding declines in resistance rates without affecting patient outcomes. Coupled with rigorous Infection Prevention and Control (IPC) protocols—hand hygiene, contact precautions, and environmental sanitation—stewardship ensures that existing drugs remain viable for as long as possible.
Innovation — Building the Future Arsenal
Bacteria evolve faster than our therapies, making innovation non-negotiable. The future of AMR control lies in the convergence of biotechnology and molecular genetics, including:
• Novel Therapeutics: The development of next-generation antibiotics (e.g., teixobactin, lefamulin) and host-directed therapies that bolster immune resilience.
• Precision Technologies: Application of genomics-based diagnostics, bacteriophage therapy, and CRISPR-Cas antimicrobials to selectively disable resistance genes within microbial genomes.
• Systems Integration: Linking real-time molecular surveillance with clinical decision systems to guide personalized, data-driven treatment strategies.
Together, stewardship and innovation form the twin pillars sustaining global antimicrobial defense—preserving today’s medicines while forging tomorrow’s breakthroughs.
Conclusion: A Coordinated Global Response
The microbial world will not stand still; resistance is an evolutionary inevitability. Yet through global cooperation, robust stewardship, and sustained innovation, it can be contained. The outcome of this struggle will define the future of medicine, agriculture, and planetary health. AMR is not solely a biomedical challenge—it is a test of humanity’s capacity to govern its own biological footprint.
About Bioguard Corporation
As part of the international effort to combat antimicrobial resistance, Bioguard Corporation remains committed to advancing veterinary diagnostics, infectious disease surveillance, and molecular testing technologies that empower clinicians and laboratories worldwide. Through the development of rapid, accurate diagnostic platforms and support for antimicrobial stewardship initiatives, Bioguard contributes to the collective mission of protecting animal and human health, preserving the efficacy of antibiotics for generations to come.
The miniAST Veterinary Antibiotic Susceptibility Test Analyzer, a tool designed to help combat antimicrobial resistance with game-changing features:
Feature | Benefit |
Fast Results | Get results in just 6 hours, enabling swift and confident treatment. |
Automated Interpretations | Instantly deliver precise susceptibility profiles, supporting faster, more informed clinical decisions and optimizing patient care. |
Dual-Sample Testing | Double the efficiency with simultaneous analysis of two samples at once. |
High Accuracy | Achieve an impressive 92% accuracy rate compared to traditional disc diffusion tests. |
📌 Note for Veterinarians:
The MiniAST Veterinary Antibiotic Susceptibility Test Analyzer is available exclusively to licensed veterinarians and veterinary hospitals.
📩 How to Order MiniAST
To purchase MiniAST or request a quotation, please contact our sales team or email our customer service:
📧 service@bioguardlabs.com
☎️ Please include your hospital name and contact number so our sales representative can follow up with you directly.
Sourse:
The provided source material consists of multiple independent documents (articles, reports, theses, and Wikipedia pages) and citation lists from within those documents. Since “vapa style” is not a standard known style, and based on the previous turn, I will provide the references in APA style (7th edition format), utilizing the full metadata found within your excerpts for each distinct source document.
References (APA Style)
- CDC Fact Sheet/Report (Antimicrobial Resistance Facts and Stats) Centers for Disease Control and Prevention. (2025, February 4). Antimicrobial resistance facts and stats. U.S. Department of Health and Human Services.
- Journal Article (An overview of the antimicrobial resistance mechanisms of bacteria) Reygaert, W. C. (2018). An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiology, 4(3), 482–501. https://doi.org/10.3934/microbiol.2018.3.482 (Note: The journal name is inferred from the DOI structure/standard academic format, as the name was not explicitly listed in the immediate excerpt header.)
- Journal Article (Classification for β-lactamases: historical perspectives) Bush, K. (2023). Classification for $\beta$-lactamases: historical perspectives. Expert Review of Anti-infective Therapy. (Specific volume/issue information is not present in the excerpt).
- Journal Article (Competence in Streptococcus pneumoniae…) Salvadori, G., Junges, R., Morrison, D. A., & Petersen, F. C. (2019). Competence in Streptococcus pneumoniae and close commensal relatives: Mechanisms and implications. Frontiers in Cellular and Infection Microbiology, 9, Article 94. https://doi.org/10.3389/fcimb.2019.00094
- Journal Article (Integrons and antimicrobial resistance in bacteria…) Shariati, A., Sabzehali, F., Goudarzi, M., & Azimi, H. (2018). Integrons and antimicrobial resistance in bacteria: A systematic review. Journal of Paramedical Sciences (JPS), 9(2), 39–48. (Page range inferred from the excerpt text.)
- Journal Article (Mechanistic Principles Behind Molecular Mechanism of Rifampicin Resistance…) Singh, A., Grover, S., Sinha, S., Das, M., Somvanshi, P., & Grover, A. (2017). Mechanistic Principles Behind Molecular Mechanism of Rifampicin Resistance in Mutant RNA Polymerase Beta Subunit of Mycobacterium tuberculosis. Journal of Cellular Biochemistry, (12), 4594–4606. https://doi.org/10.1002/jcb.26124
- Journal Article (Molecular Mechanisms of Antimicrobial Resistance…) Dominic Terkimbi, S., Aja Maduabuchi, P., Chans Mwandah, D., Danchal Vandu, C., A. B, A., Paul-Chima, U. O., Samson Dangana, R., & Mujinya, R. (2025). Molecular Mechanisms of Antimicrobial Resistance in Infectious Diseases: Advances in Therapeutic Strategies and Public Health Interventions [version 1; peer review: 1 approved with reservations]. F1000Research, 14, Article 783. https://doi.org/10.12688/f1000research.167514.1
- Journal Article (The Role of Efflux Pumps in Antibiotic Resistance…) Zhan, E. (2025). The role of efflux pumps in antibiotic resistance among Gram-Negative bacteria. Journal of Infectious Diseases and Prevention. https://doi.org/10.4172/jidp.1000281 (Note: The journal name “JIDP” is inferred from the provided DOI and abbreviation.)
- Journal Article (Staphylococcus aureus temperate bacteriophage…) McCarthy, A. J., Witney, A. A., & Lindsay, J. A. (2012). Staphylococcus aureus temperate bacteriophage: carriage and horizontal gene transfer is lineage associated. Frontiers in Cellular and Infection Microbiology, 2, Article 6. https://doi.org/10.3389/fcimb.2012.00006
- Journal Article (Spontaneous Mutations That Confer Antibiotic Resistance in Helicobacter pylori) Wilson, T. J. M., Jiang, Q., & Taylor, D. E. (n.d.). Spontaneous mutations that confer antibiotic resistance in Helicobacter pylori. [Journal Information Missing].
- Review Article (Transmission of antibiotic resistance genes…) He, J., Yan, Z., & Chen, Q. (n.d.). Transmission of antibiotic resistance genes in agroecosystems: an overview. [Journal Information Missing].
- Thesis (Conjugative plasmids…) Mølbak, L. (2003). Conjugative plasmids and the transfer of mobile genetic elements among bacteria in plant rhizosphere environments [Ph.D. thesis, The Technical University of Denmark]. National Environmental Research Institute. http://www.dmu.dk/1_viden/2_Publikationer/ovrige/rapporter/phd_lam.pdf
- Reference Cited in Source 14 (IDID: Inherited Diseases in Dogs…) Sargan, D. R. (2004). IDID: Inherited Diseases in Dogs: Web-based information for canine inherited disease genetics. Mammalian Genome, 15(6), 503–506. https://doi.org/10.1007/s00335-004-3047-z
- Reference Cited in Source 16 (Dissemination of the mcr-1 colistin resistance gene) Olaitan, M. O., Chabou, S., Okdah, L., Morand, S., & Rolain, J. M. (2016). Dissemination of the mcr-1 colistin resistance gene. Lancet Infectious Diseases, 16(2), 147. https://doi.org/10.1016/S1473-3099(15)00540-X
- Encyclopedia Entry (Beta-lactamase) Beta-lactamase. (n.d.). In Wikipedia. Retrieved from [URL of Wikipedia page is not provided in the source].
