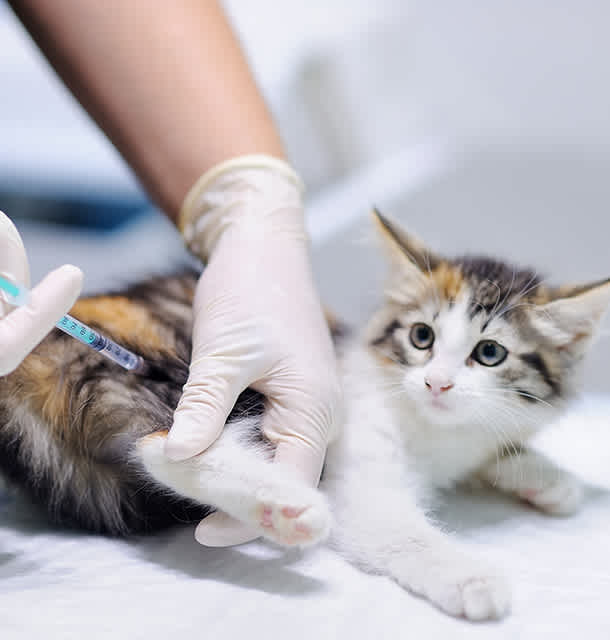
Bioguard Showcases miniAST at Veterinary Seminar in Jakarta, Indonesia

In Jakarta, Indonesia, Bioguard launched the miniAST Veterinary Antibiotic Susceptibility Test Analyzer during the seminar “Optimizing Antibiotics: Tips for Choosing the Best Drug, Dose, Route, and Duration”, attended by Indonesian veterinarians, showcasing its commitment to evidence-based antimicrobial use. At the seminar, veterinarians were introduced to miniAST, a compact, clinic-friendly analyzer designed to make antibiotic susceptibility testing faster and more accessible. Bioguard demonstrated how miniAST can deliver susceptibility results in as little as 6 hours, with accuracy up to 92% compared to traditional disc diffusion, all while fitting seamlessly into standard small-animal clinic workflows. Interactive Discussions and Q&A Following the product presentation, participants engaged in interactive discussions and a Q&A session. Topics included strategies for choosing the right drug, optimizing dose and duration, and improving stewardship in environments where empirical antibiotic use is still common. This exchange allowed veterinarians to explore practical approaches for reducing antimicrobial resistance (AMR) in companion animals while integrating new diagnostic tools like miniAST into their clinics. AMR Trends in Southeast Asia Antimicrobial resistance is an emerging challenge in companion-animal medicine across Southeast Asia, with veterinarians reporting an increase in recurrent and difficult-to-treat infections in dogs and cats. Studies from countries including Thailand, Singapore, Malaysia, and Indonesia have identified multidrug-resistant organisms such as ESBL-producing Escherichia coli and methicillin-resistant Staphylococcus species (MRSA, MRSP) in companion animals. Limited access to routine antibiotic susceptibility testing means that antibiotic treatment is often empirical, with broad-spectrum antibiotics commonly used as first-line therapy. Combined with variable awareness of appropriate antibiotic use among pet owners, these factors contribute to sustained selection pressure and the continued spread of resistant bacteria, underscoring the need for improved stewardship and accessible in-clinic diagnostics. Antimicrobial Resistance (AMR) in Indonesia Antimicrobial resistance (AMR) is an increasing concern in Indonesia, driven by widespread antimicrobial use across human health, veterinary practice, and agriculture. Antibiotics critical to human medicine, including penicillins, tetracyclines, fluoroquinolones, and sulfonamides, are commonly used in animal health, often prescribed empirically due to limited access to routine diagnostics and antimicrobial susceptibility testing (AST). Studies have reported multidrug-resistant bacteria in production animals, companion animals, and stray populations, underscoring the risk of resistance transmission across sectors. Indonesia has responded through the National Action Plan on AMR (NAP AMR) 2020–2024, aligned with the WHO Global Action Plan and based on a One Health approach. The strategy promotes coordinated action across human, animal, food, and environmental sectors, with support from FAO, WHO, and WOAH. Key actions include restrictions on antibiotic growth promoters, strengthened AMR surveillance, increased private-sector engagement, and policies encouraging responsible antimicrobial use. Despite progress at the national level, challenges remain in implementation at local and clinical levels. Limited diagnostic capacity, uneven adoption of antimicrobial stewardship practices, and fragmented surveillance systems continue to drive reliance on broad-spectrum antibiotics. Expanding access to veterinary diagnostics, strengthening stewardship, and improving cross-sector data integration are essential to sustaining AMR control and protecting both animal and public health in Indonesia. Future Commitment to Veterinary Experts By showcasing miniAST at the Veterinary Seminar in Jakarta, Indonesia, we continue our mission of advancing veterinary diagnostics across ASEAN and beyond. In clinical settings where empirical antibiotic use is still common, miniAST provides veterinarians with fast, actionable antibiotic susceptibility results, helping them make more confident and precise treatment decisions. By reducing reliance on broad-spectrum antibiotics, it integrates precision prescribing into everyday practice, addressing the unique challenges ASEAN veterinarians face while promoting responsible antibiotic stewardship and improving patient care. The miniAST Veterinary Antibiotic Susceptibility Test Analyzer, is a solution designed to help combat antibiotic resistance with game-changing features: Feature & Benefit Feature Benefit Fast Results Get results in just 6 hours, enabling swift and confident treatment. Automated Interpretations Instantly deliver precise susceptibility profiles, supporting faster, more informed clinical decisions and optimizing patient care. Dual-Sample Testing Double the efficiency with simultaneous analysis of two samples at once. High Accuracy Achieve an impressive 92% accuracy rate compared to traditional disc diffusion tests. 📌 Note for Veterinarians: The miniAST Veterinary Antibiotic Susceptibility Test Analyzer is available exclusively to licensed veterinarians and veterinary hospitals. 📩 How to Order miniAST To purchase miniAST or request a quotation, please contact our sales team or email our customer service: 📧 service@bioguardlabs.com Please include your hospital name and contact number so our sales representative can follow up with you directly. Stay connected with Bioguard! Follow us on social media to get the latest product updates, educational content, webinar info, and event news: Instagram: @bioguard.animalcare Facebook: @bioguardca LinkedIn: @bioguardtaiwan Instagram Facebook Linkedin
Combating AMR in Asia-Pacific: miniAST at the Center of FASAVA 2025

AMR Trends in the Asia-Pacific Region Antimicrobial resistance is becoming an increasing concern in companion-animal medicine across the Asia-Pacific, with veterinarians reporting more recurrent and difficult-to-treat infections. Recent studies have documented the rise of multidrug-resistant organisms such as ESBL-producing E. coli, MRSA, and MRSP in Thailand, Korea, and Malaysia, indicating a growing presence of resistant pathogens in dogs and cats (Satchasataporn et al., 2025; Seo et al., 2025; Afshar et al., 2023). A major contributor to this trend is the reliance on empirical antibiotic prescribing, driven by limited access to rapid diagnostic testing and low awareness of proper antibiotic use among pet owners. Research from Korea and Singapore shows that broad-spectrum antibiotics are often prescribed in the absence of timely AST results, reinforcing patterns that accelerate resistance (Kim et al., 2025). These challenges highlight the need for practical in-clinic tools that enable faster, evidence-based treatment decisions. Insights from Korean Veterinary Industry Leaders Mr. Kevin Ha, Vice President of PostBio Co., Ltd During the interview, Mr. Kevin Ha shared that antibiotic misuse is becoming increasingly serious in veterinary hospitals, similar to trends seen in human medicine. He explained that when antibiotic susceptibility testing is not performed, veterinarians often choose stronger antibiotics because they cannot confirm which drugs are effective. Antibiotic abuse is becoming more serious in the animal hospital market. When antibiotic testing is not done, strong antibiotics are often prescribed, and I believe this problem will continue to become more serious. Mr. Kevin Ha Vice President of PostBio Co., Ltd Upon being introduced to miniAST, he expressed strong optimism. He emphasized that traditional AST required 4–6 days, while miniAST provides results much more quickly. When I first saw miniAST, I thought, “This is the product I’ve been looking for.” It was impressive and hopeful. Previously, AST took at least 4-6 days, but with this product, the results come out very fast. It is extremely helpful, and we have high expectations. Mr. Kevin Ha Vice President of PostBio Co., Ltd Dr. Kyu Wook Kim, President of Solvet, Inc. Dr. Kim highlighted the growing global threat of antimicrobial resistance and referenced a widely cited projection. He stressed that antibiotics must be used carefully. As far as I know, by 2050, AMR is expected to cause more deaths than cancer. Antibiotics should be used properly and responsibly. They should not be used indiscriminately as they often are now. Dr. Kyu Wook Kim President of Solvet, Inc. When he first saw miniAST, he was surprised by its speed and practicality. I was very surprised. In the past, sending samples to a lab service took about 5–6 days including preparation, but miniAST provides results in about six hours. It is extremely efficient, and I believe equipment like this is absolutely necessary for the health of both humans and animals. Dr. Kyu Wook Kim President of Solvet, Inc. Dr. Kim also noted that the adoption of such devices in Korean clinics is steadily increasing, and broader use will help reduce unnecessary antibiotic prescriptions. If these devices become more widely used, we can reduce unnecessary antibiotic use and lower AMR-related risks. Dr. Kyu Wook Kim President of Solvet, Inc. Antimicrobial Resistance in the Republic of Korea Antimicrobial resistance (AMR) represents an important public health concern in Korea, where antibiotic consumption remains relatively high. According to the OECD, the daily defined doses of antibiotic prescriptions per 1,000 population in the Republic of Korea are among the highest reported across OECD member countries, indicating a potential risk for the development and spread of antimicrobial resistance (OECD, 2023; OECD, 2025). In response to AMR, the Republic of Korea established the First National Action Plan on Antimicrobial Resistance (2016–2020) based on the WHO Global Action Plan on AMR. This national strategy adopted a multi-sectoral approach encompassing human health, animal health, food safety, and environmental sectors. Within the animal health sector, key measures have included the strengthening of AMR surveillance systems and the introduction of antimicrobial stewardship program (ASP) pilot projects, aimed at promoting appropriate antimicrobial use and reducing antimicrobial resistance. The One Health AMR concept, which connects human, animal, and environmental health, has gained increasing attention across medical, veterinary, and environmental communities in the Republic of Korea. Korean veterinary medical societies have made significant efforts to minimize the potential impact of animal antimicrobial use on public and animal health. Studies and professional discussions indicate that many Korean veterinarians recognize the risks associated with the misuse or overuse of antibiotics and are considering the development of improved guidelines and regulatory measures. About the miniAST Veterinary AST Analyzer The Bioguard miniAST Veterinary Antibiotic Susceptibility Test Analyzer is an automated system designed specifically for veterinary antimicrobial susceptibility testing. It provides: Categorical susceptibility results (susceptible, intermediate, resistant) Automated interpretation to minimize human error Results within approximately six hours, enabling same day therapeutic adjustments Standardized, reproducible data to guide confident antibiotic selection By shortening the time between diagnosis and targeted therapy, miniAST helps veterinary clinics avoid unnecessary or ineffective antibiotic use, supporting responsible antimicrobial stewardship and improving clinical outcomes. References Afshar, M. F., Zakaria, Z., Cheng, C. H., & Ahmad, N. I. (2023). Prevalence and multidrug-resistant profiles of methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus pseudintermedius in dogs, cats, and pet owners in Malaysia. Veterinary World, 16(3), 536–545. https://doi.org/10.14202/vetworld.2023.536-545 Kim, B., Kim, Y. J., & Lee, H. (2025). National action plan on antimicrobial resistance in the Republic of Korea: Progress and challenges. Infection & Chemotherapy, 57(1), 1–12. https://doi.org/10.3947/ic.2025.0028 Kim, S.-M., Kim, H.-S., Kim, J.-W., & Min, K.-D. (2025). Assessment of antimicrobial use for companion animals in the Republic of Korea: Developing defined daily doses and investigating veterinarians’ perception of antimicrobial resistance. Animals, 15(2), 260. https://doi.org/10.3390/ani15020260 Lee, J., Kim, D., & Choi, Y. (2025). Antimicrobial use and resistance in companion animals in Korea. Animals, 15(2), 260. https://www.mdpi.com/2076-2615/15/2/260 Organisation for Economic Co-operation and Development. (2023). Health at a glance 2023: OECD indicators. OECD Publishing. https://www.oecd.org/en/publications/health-at-a-glance-2023_8f9e3f98-en.html Organisation for Economic Co-operation and Development. (2025). Health at a glance 2025. OECD Publishing. https://www.oecd.org/en/publications/health-at-a-glance-2025_8f9e3f98-en.html Park, Y. H. (n.d.). One Health approach to
Bioguard Showcases miniAST at FASAVA 2025 in Daegu, Korea

From October 31 to November 2, 2025, Bioguard joined the 13th FASAVA Congress at EXCO in Daegu, Korea (R.O.K.), one of the key small animal veterinary events in the Asia-Pacific. Under the congress theme “One Vision, One Voice: Advancing Asia Pacific Veterinary Medicine” Bioguard showcased the miniAST Veterinary Antibiotic Susceptibility Test Analyzer as part of its commitment to smarter, evidence-based antimicrobial use in daily practice. Bringing miniAST to FASAVA 2025 At the Bioguard booth, veterinarians from across Asia and beyond were introduced to miniAST, a compact, clinic-friendly analyzer designed to make antibiotic susceptibility testing both faster and more accessible. Building on campaigns that highlighted miniAST’s speed and practicality, Bioguard demonstrated how the system can deliver susceptibility results in as little as 6 hours, with up to 92% accurate compared to traditional disc diffusion, while fitting easily into typical small-animal clinics. Expert Insights on miniAST Mr. Kevin Ha (Vice President of PostBio Co., Ltd) Mr. Kevin Ha shared that his first impression of miniAST was extremely positive, describing it as exactly the solution the market has been waiting for. He praised how the system dramatically shortens AST turnaround time, reducing the traditional 4–6 day workflow to just a few hours. He noted that this improvement makes miniAST highly practical and clinically impactful for veterinarians. Overall, he expressed strong optimism about its future adoption and usefulness in animal hospitals. Dr. Kyu Wook Kim, Ph.D. (President of Solvet, Inc) Dr. Kim expressed surprise and admiration when he first saw miniAST, highlighting its efficiency compared to conventional laboratory AST, which typically requires 5–6 days. He emphasized that miniAST provides reliable results in approximately 6 hours, offering a major improvement in diagnostic speed. He believes such in-clinic tools will greatly benefit veterinarians by simplifying workflows and enhancing treatment decision-making. Dr. Kim also noted that miniAST represents an essential advancement for modern veterinary practice. Looking ahead to FASAVA 2026 in Taipei As FASAVA 2025 came to a close in Daegu, excitement began building for FASAVA 2026 in Taipei, the next official host city and home of Bioguard. In 2026, Bioguard looks forward to welcoming regional and international veterinary experts and industry professionals to our home city, where we will further demonstrate how miniAST integrates into a comprehensive diagnostic portfolio supporting antimicrobial stewardship in daily practice. We remain committed to providing practical, evidence-based tools that help veterinarians make informed decisions and enhance patient care. Through its presence at FASAVA 2025, Bioguard underscored a clear message: antibiotic susceptibility testing is becoming more accessible than ever, and with innovations like miniAST, small-animal clinics can take a more proactive, data-driven approach to combating AMR. We look forward to meeting veterinarians, partners, and industry professionals in Taipei for FASAVA 2026, where Bioguard will continue advancing the future of veterinary diagnostics in the Asia-Pacific region. Future Commitment to Veterinary Experts By showcasing miniAST at the 13th FASAVA Congress at EXCO in Daegu, we strengthen our mission to elevate veterinary diagnostics and support veterinarians around the world. Our focus remains on delivering innovative solutions that help professionals offer better care and manage antibiotic resistance effectively. The miniAST Veterinary Antibiotic Susceptibility Test Analyzer, a tool designed to help combat antimicrobial resistance with game-changing features: Feature & Benefit Feature Benefit Fast Results Get results in just 6 hours, enabling swift and confident treatment. Automated Interpretations Instantly deliver precise susceptibility profiles, supporting faster, more informed clinical decisions and optimizing patient care. Dual-Sample Testing Double the efficiency with simultaneous analysis of two samples at once. High Accuracy Achieve an impressive 92% accuracy rate compared to traditional disc diffusion tests. 📌 Note for Veterinarians: The miniAST Veterinary Antibiotic Susceptibility Test Analyzer is available exclusively to licensed veterinarians and veterinary hospitals. 📩 How to Order miniAST To purchase miniAST or request a quotation, please contact our sales team or email our customer service: 📧 service@bioguardlabs.com ☎️ Please include your hospital name and contact number so our sales representative can follow up with you directly. https://fasava2025.org/
AMR Rise in Latin America: Insights from WSAVA 2025 and miniAST Solutions

The Growing AMR Challenge in Latin America Antimicrobial resistance (AMR) has emerged as one of the most critical health threats in both human and veterinary medicine. In the Latin American region, widespread antibiotic access, inconsistent treatment compliance, and limited diagnostic resources have accelerated resistance, complicating treatment options for everyday clinical cases. At the WSAVA Congress 2025 in Rio de Janeiro, Bioguard engaged with veterinary experts from Paraguay to explore how resistance is affecting regional clinical practice and how new diagnostic tools, the miniAST Veterinary Antibiotic Susceptibility Test Analyzer, can help veterinarians make evidence based treatment decisions faster. Insights from Paraguayan Veterinary Experts Dr. Carlos Cabrera, a veterinarian specializing in oncology and cytology, emphasized that antimicrobial resistance is already a major regional health concern, particularly the rapid rise of antibiotic resistance across clinical cases. Antibiotic resistance is becoming extremely important. Around 10 million people die each year due to antibiotic resistance, and in the next 15 to 20 years this number could rise to 39 million. Dr. Carlos Cabrera He explained that resistant infections increasingly complicate therapy in routine cases, underscoring the urgency of adopting faster diagnostic approaches. He emphasized the value of rapid antibiotic susceptibility testing: miniAST gives us a quick solution about which antibiotic to use, helping us avoid those that are resistant. It allows us to make treatment decisions based on evidence, not assumption. Dr. Carlos Cabrera Dr. Fabián Velázquez, a small animal veterinarian from San Lorenzo, Paraguay, described the real world challenges of AMR in clinical practice. When we perform an antibiogram culture, we see very high levels of resistance. Sometimes we hesitate because we don’t know which antibiotic will work, or we need to start combining drugs. It is extremely concerning. Dr. Fabián Velázquez He also highlighted the broad clinical utility of rapid AST: miniAST will help me every day in the clinic. It allows us to focus our diagnosis and treatment much better. Dr. Fabián Velázquez He emphasized the broad utility of rapid diagnostics across multiple clinical areas: I can use it in dentistry, dermatology, traumatic surgery, even cavity infections. It will help daily and in a very short time. Dr. Fabián Velázquez Both veterinarians agreed: rapid AST tools are not only convenient, they are becoming essential for accurate therapy, reduced empirical prescribing, and minimizing treatment failures. AMR in Latin America: The Regional Picture According to data from the Pan American Health Organization (PAHO), resistance rates among pathogens such as Escherichia coli, Staphylococcus aureus, and Klebsiella pneumoniae have been steadily increasing across Latin America. In veterinary medicine, this trend poses a direct threat to animal health, especially in regions with limited laboratory capacity and where empirical treatment remains common. Key factors driving AMR in Latin America include: Widespread availability of antibiotics without prescription Limited surveillance of regional bacterial resistance Delayed diagnostic confirmation in clinical cases Overlapping antibiotic use in both human and veterinary sectors This creates a dangerous feedback loop in which the lack of diagnostic testing leads to greater reliance on empirical therapy, driving increased resistance and ultimately resulting in more frequent treatment failures. Dr. Cabrera captured this concern clearly: When we treat animals without knowing the bacterial profile, we risk worsening resistance. It’s not just a veterinary issue, it’s a public health issue. Dr. Cabrera One Health Approach: miniAST as a Diagnostic Bridge The One Health initiative emphasizes the interdependence of human, animal, and environmental health. Veterinarians play a crucial role by ensuring antibiotics are used responsibly and only when supported by diagnostic evidence. By providing rapid, clinic based AST, miniAST bridges the diagnostic gap between clinical need and laboratory confirmation. It strengthens evidence based therapy, reduces unnecessary antibiotic exposure, and supports regional AMR surveillance efforts. Dr. Velázquez described the broader potential: If we could test every infection this way, we would reduce unnecessary antibiotic exposure. It would help veterinarians, owners, and even policymakers understand how to control resistance in our region. Dr. Velázquez About the miniAST Veterinary AST Analyzer The Bioguard miniAST Veterinary Antibiotic Susceptibility Test Analyzer is an automated system designed specifically for veterinary antimicrobial susceptibility testing. It provides: Categorical susceptibility results (susceptible, intermediate, resistant) Automated interpretation to minimize human error Results within approximately six hours, enabling same day therapeutic adjustments Standardized, reproducible data to guide confident antibiotic selection By shortening the time between diagnosis and targeted therapy, miniAST helps veterinary clinics avoid unnecessary or ineffective antibiotic use, supporting responsible antimicrobial stewardship and improving clinical outcomes. The miniAST Veterinary Antibiotic Susceptibility Test Analyzer, a tool designed to help combat antimicrobial resistance with game-changing features: Feature Benefit Fast Results Get results in just 6 hours, enabling swift and confident treatment. Automated Interpretations Instantly deliver precise susceptibility profiles, supporting faster, more informed clinical decisions and optimizing patient care. Dual-Sample Testing Double the efficiency with simultaneous analysis of two samples at once. High Accuracy Achieve an impressive 92% accuracy rate compared to traditional disc diffusion tests. 📌 Note for Veterinarians:The miniAST Veterinary Antibiotic Susceptibility Test Analyzer is available exclusively to licensed veterinarians and veterinary hospitals. 📩 How to Order miniASTTo purchase miniAST or request a quotation, please contact our sales team or email our customer service:📧 service@bioguardlabs.com☎️ Please include your hospital name and contact number so our sales representative can follow up with you directly. References Pan American Health Organization (PAHO). Antimicrobial Resistance in the Americas: Epidemiological Update 2021–2022. Washington, DC. World Health Organization (WHO). Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022. Geneva: WHO. PAHO. One Health and Antimicrobial Resistance Technical Brief. 2022. Rodríguez-Medina N, et al. “Antimicrobial Resistance in Latin America: Current Status and Challenges.” Journal of Global Antimicrobial Resistance. 2021. López-Jiménez L, et al. “Antibiotic Use and Resistance in Latin America: A Systematic Review.” Infection Control & Hospital Epidemiology. 2020.
Bioguard Partners with Nu.Q® by Volition to Advance Canine Cancer Screening

Bioguard Reference Laboratory has long been recognized as a trusted leader in veterinary diagnostics. With ISO/IEC 17025 accreditation and BSL-2 laboratory certification, Bioguard proudly holds the distinction of being Taiwan’s first accredited animal disease testing laboratory. Equipped with advanced PCR technology and automated high-throughput systems, the laboratory delivers fast, precise, and reliable results to veterinarians. Supported by 24-hour client service, Bioguard remains dedicated to empowering veterinary professionals through innovative and dependable diagnostic solutions. Driven by a mission to elevate global diagnostic standards, Nu.Q® by Volition has established a partnership with Bioguard, recognizing the laboratory’s strong reputation, technical expertise, and proven reliability in veterinary diagnostics. This partnership reflects a shared vision: to make early cancer detection more accessible and precise for veterinarians worldwide. The collaboration between Bioguard and Nu.Q® by Volition grew naturally from shared values in advancing veterinary diagnostics and improving animal health. During their visit to Bioguard’s Reference Laboratory in Taiwan, Nu.Q® representatives observed the laboratory’s daily operations and commitment to quality. The visit provided an opportunity for open professional exchange, allowing both teams to discuss workflow practices, diagnostic standards, and the potential for integrating the Nu.Q® Vet Cancer Test into Bioguard’s existing services. Following the visit, the Nu.Q® team provided comprehensive technical training to Bioguard’s laboratory members, helping to ensure precise testing procedures and consistent interpretation of results. Through these discussions and hands-on sessions, both sides developed a deeper understanding of each other’s expertise and a shared sense of purpose in bringing reliable cancer screening tools to veterinarians. Nu.Q® can help veterinarians and owners understand a dog’s current physical condition and detect cancer at an early stage. If you are a dog owner who cares about your dog’s health, we encourage including the Nu.Q® Vet Cancer Test as part of your pet’s annual wellness exam. Through Bioguard’s Reference Laboratory, veterinarians can now access this testing service to support early cancer screening. This allows both pet owners and veterinarians to gain a clearer, more comprehensive understanding of a dog’s overall health. Dr. Peggy Lan DVM Technical Support Specialist, Bioguard Corporation The collaboration began when Nu.Q® by Volition recognized Bioguard’s strong reputation, technical expertise, and laboratory capabilities, making it an ideal partner to implement their advanced canine cancer test in Taiwan. Through professional discussions, training sessions, and shared values in advancing veterinary medicine, both teams identified a shared goal: to combine global innovation with local expertise. About Nu.Q® by Volition The Nu.Q® Vet Cancer Test is a cutting-edge, non-invasive blood test for dogs that detects circulating nucleosomes, DNA-protein fragments released into the bloodstream during cancer and cell death. Designed as an early cancer screening and monitoring tool, it is particularly recommended for senior dogs (over seven years old) and breeds predisposed to cancer. 🧑⚕️ Nu.Q® Vet Cancer Test – Veterinarians – Volition Volition Continues to Extend Access to Nu.Q® Vet Cancer Test Explore our comprehensive diagnostic testing services tailored for veterinary professionals. At Bioguard, we conduct advanced molecular diagnostics, endocrinology, hematology/chemistry, genetic testing, allergen screening, bacteriology, and histopathology/cytology in one centralized laboratory. Visit our Diagnostic Testing Services to discover how we support precise and evidence-based diagnoses for veterinarians. 🔗https://www.bioguardlabs.com/diagnostic-testing-services/
Bioguard Launches miniAST at WSAVA Congress 2025 in Rio de Janeiro, Brazil

Showcasing Bioguard Veterinary Diagnostics Products From September 25–27, 2025, at the WSAVA Congress in Rio de Janeiro, Brazil, Bioguard proudly presented the miniAST Veterinary Antibiotic Susceptibility Test Analyzer. miniAST provides rapid, precise antibiotic susceptibility testing, helping veterinarians make informed treatment decisions while supporting the global fight against antimicrobial resistance (AMR). Engaging with Veterinary Professionals During WSAVA Congress 2025 in Rio, Bioguard’s booth welcomed veterinary professionals from around the world, all eager to experience miniAST firsthand. Through live demonstrations, visitors could see the device’s speed, accuracy, and ease of use in action. Attendees asked questions, explored practical applications, and discovered how miniAST can make diagnostics more efficient and reliable in everyday clinical practice. Among the experts who visited the booth were: Dr. Ernesto Bruzzone from the Instituto Gastroenterológico Veterinario, Argentina. He is a leading veterinarian and founder of the Institute of Veterinary Gastroenterology, which for 27 years has educated professionals across Latin America through congresses, diploma programs, and internships. He earned his Veterinary Medicine degree at the National University of La Plata and later specialized in Endoluminal Endoscopy and Minimally Invasive Surgery in Spain. In addition, he teaches postgraduate courses and frequently speaks at national and international conferences. We also had the opportunity to interview veterinarians, including professionals from Paraguay, gaining valuable insights from their experiences and perspectives on advancing veterinary care in the region. Dr. Fabian Velazquez Dr. Fabián Velázquez, a 2016 graduate of the National University of Asunción, is a veterinarian committed to excellence in animal care, with specializations in University Teaching, Veterinary Ultrasound, and Small Animal Clinical Laboratory work. He sees animals as cherished family members, providing compassionate, personalized care and long-term well-being for each patient. Beyond the clinic, he is active in his community as a board member of the Asociación Joven Rural del Paraguay and serves as Paraguay’s Ambassador for FORVET, while also participating in K9 search dog training programs. http://agroclinicacapital.agenciawebporta.com/ These interactions offered valuable insights into clinical challenges and the practical applications of miniAST in veterinary practice. Dr. Carlos Cabrera Dr. Carlos Cabrera is a veterinarian who graduated in 2019 from the National University of Asunción. Passionate about veterinary oncology and cytology, he holds a certification in oncology and dedicates his career to the comprehensive care of pets. He firmly believes that our animals are part of the family and that, even when a cure is not possible, it is always possible to care for them with love, respect, and commitment. Driving Responsible Antibiotic Use miniAST allows veterinarians to quickly identify the most effective antibiotics, helping reduce unnecessary or ineffective treatments. This not only leads to better outcomes for patients but also supports responsible antimicrobial stewardship, an important step in the global fight against antimicrobial resistance (AMR). Future Commitment to Veterinary Experts By showcasing miniAST at WSAVA Congress, we reinforce our dedication to advancing veterinary diagnostics and supporting veterinarians worldwide. We remain committed to providing innovative solutions that enable professionals to deliver high-quality care while addressing the challenges of antimicrobial resistance. The miniAST Veterinary Antibiotic Susceptibility Test Analyzer, a tool designed to help combat antimicrobial resistance with game-changing features: Feature Benefit Fast Results Get results in just 6 hours, enabling swift and confident treatment. Automated Interpretations Instantly deliver precise susceptibility profiles, supporting faster, more informed clinical decisions and optimizing patient care. Dual-Sample Testing Double the efficiency with simultaneous analysis of two samples at once. High Accuracy Achieve an impressive 92% accuracy rate compared to traditional disc diffusion tests. 📌 Note for Veterinarians: The miniAST Veterinary Antibiotic Susceptibility Test Analyzer is available exclusively to licensed veterinarians and veterinary hospitals. 📩 How to Order miniAST To purchase miniAST or request a quotation, please contact our sales team or email our customer service: 📧 service@bioguardlabs.com ☎️ Please include your hospital name and contact number so our sales representative can follow up with you directly. https://wsava-congres13/
Comprehensive Clinical Approach to Bacterial Respiratory Infections in Cats and Dogs

Table of Contents Overview of Bacterial Respiratory Infections in Dogs and Cats Bacterial respiratory infections (BRIs) are a key concern for veterinary clinicians, affecting both dogs and cats. They can range from mild upper respiratory disease to severe, sometimes fatal, lower respiratory infections. Common causative pathogens include: Bordetella bronchiseptica: a major pathogen in canine infectious respiratory disease complex (CIRDC) Mycoplasma species: frequently linked to chronic respiratory infections in dogs Chlamydia felis: a primary cause of conjunctivitis and upper respiratory disease in cats Neisseria animaloris: normally a commensal in the upper respiratory tract, but can occasionally cause pulmonary infections These pathogens cause inflammation and damage within the respiratory tract, making animals more susceptible to secondary infections and long-term complications. Clinical Signs and Diagnosis The clinical presentation depends on the location and severity of infection: Upper respiratory tract infections: coughing, sneezing, nasal and ocular discharge, lethargy, reduced appetite Lower respiratory tract infections: labored breathing, tachypnea, coughing, cyanosis, fever, wheezes, or crackles on auscultation Severe cases: particularly in young or immunocompromised animals, may progress to pneumonia, causing significant morbidity and mortality Diagnostic Approach A systematic approach is critical: Collect history and perform physical examination to assess onset, duration, progression, and exposure risk Use imaging such as radiographs or ultrasonography to evaluate lung involvement, pleural effusion, or consolidations Conduct laboratory tests including complete blood count and serum biochemistry to assess systemic health Perform bronchoalveolar lavage to collect lower respiratory tract samples Utilize microbiological analysis, culture and sensitivity, or PCR testing to identify causative bacterial pathogens Treatment and Supportive Care Empirical Antibiotic Therapy: Broad-spectrum antibiotics such as doxycycline may be started when the causative pathogen is unknown. Targeted Adjustments: Once culture or PCR results are available, refine the treatment plan to specifically address the identified pathogen, helping to prevent the development of antimicrobial resistance. Supportive Care: Fluid therapy to maintain hydration Oxygen supplementation for hypoxic animals Nutritional support/assisted feeding for patients with poor appetite Environmental control: isolate infected animals, and maintain proper ventilation, humidity, and air quality to ease respiratory distress Preventive Measures Vaccination: protects against Bordetella bronchiseptica in dogs and Chlamydia felis or Bordetella in cats Hygiene: regular cleaning, disinfection, and litter maintenance Isolation: separate new or sick animals in multi-pet households or shelters A comprehensive approach such as accurate diagnosis, targeted treatment, and preventive measures allows veterinary clinicians to effectively manage BRIs, reduce morbidity, and improve outcomes in dogs and cats. To help veterinary clinicians accurately diagnose respiratory infections in dogs and cats, Bioguard offers Qmini Real-time PCR Series, designed to provide fast, reliable, and actionable results. 🔎 Recommended Qmini Real-time PCR Analyzer Panels for Suspected Respiratory Infections Canine Respiratory Panel Product Name Pathogens Detected Veterinary Use Canine Respiratory Panel (4 items) Canine Distemper Virus / Canine Adenovirus Type II / Canine Parainfluenza Virus / Bordetella spp. Provides fast and accurate molecular detection of key respiratory pathogens, supporting precise diagnosis and targeted treatment in dogs Feline Respiratory Panel Product Name Pathogens Detected Veterinary Use Feline Respiratory Panel (4 items) Feline Calicivirus / Feline Herpesvirus / Chlamydia felis / Mycoplasma felis Enables rapid identification of common feline respiratory pathogens, assisting veterinarians in accurate diagnosis and management 📌 Note for Veterinarians:For purchase, please contact our sales team or customer service to receive professional consultation and ordering details. 📩 How to Order All rapid test kits listed above are available exclusively to licensed veterinarians and veterinary hospitals. To place an order or request a quotation, please contact our sales team or email our customer service: 📧 service@bioguardlabs.com☎️ Please include your hospital name and contact number in the email so our sales representative can follow up with you directly.
Unravelling the Challenge: In-Clinic Diagnosis of the Deadly Feline Infectious Peritonitis (FIP)

Table of Contents What is FIP? Feline Infectious Peritonitis (FIP) is one of the hardest diseases to diagnose in cats.It is caused by a mutation of the common feline coronavirus (FCoV). In most cats, this virus only causes mild or unnoticed gut infections. But in some cats, it mutates and develops into FIP. Forms of the Disease FIP appears in two forms: effusive (wet) and non-effusive (dry). Wet FIP causes fluid build-up in body cavities. This leads to a swollen belly (abdominal distension) or fluid in the chest (pleural effusion). Dry FIP causes lumps of inflammation in organs such as the eyes, brain, or kidneys. This often shows up as neurological or eye-related problems. Because symptoms vary so much, FIP can be difficult to recognize. How Vets Make a Diagnosis Diagnosis depends on combining the cat’s history, age, breed, clinical signs, and lab tests. Young, purebred cats are more commonly affected.Nonspecific signs include long-lasting fever, weight loss, and low energy. If normal treatments do not work, vets may suspect FIP. Blood tests often show changes such as: High globulin levels High total protein Low albumin-to-globulin ratio Sometimes, low lymphocyte counts These changes support a diagnosis but do not prove FIP.In wet FIP, testing the fluid can provide important clues. Fluid is often high in protein but low in cells. The Rivalta test can be done quickly in the clinic, but it is not very specific. Confirming the Disease Definitive tests include RT-PCR to detect FCoV RNA, and tissue tests (immunohistochemistry or immunofluorescence) to show viral antigens. These tests are especially useful in dry FIP cases.However, PCR cannot always tell the difference between harmless FCoV and the FIP-causing type. Results must always be interpreted alongside lab and clinical findings. Imaging, such as ultrasound or MRI, can help find affected organs or fluid. These results also guide vets on where to collect samples for cytology or histopathology. The Challenge of Diagnosis In practice, diagnosing FIP is rarely straightforward. It requires combining evidence from clinical signs, lab tests, and imaging, rather than relying on a single test.Vets must balance the urgency of treatment with the limits of current tools. The Future of FIP Diagnosis A definitive diagnosis of FIP in practice is rarely certain. Instead, it is a matter of probability that depends on combining clinical signs, lab results, and imaging, rather than relying on one single test. Vets must balance the urgency of treatment with the limits of current diagnostic tools, weighing both supportive and confirmatory findings to guide their decisions. Ongoing research into rapid antigen tests, point-of-care PCR, and new biomarkers may improve both the speed and accuracy of FIP diagnosis. These advances could give veterinarians more reliable tools to manage this consistently serious disease. Reference: GSEPEM. (n.d.). [Image from website]. GSEPEM. https://gsepem.com/?g=404111816 🔎 Recommended Screening Products for Suspected FIP Cases Product Name Purpose in FIP Diagnosis Veterinary Use FCoV Ab Test Detects exposure to feline coronavirus (FCoV), the precursor virus of FIP. Helps identify cats at risk of mutation into FIP. FCoV Ag Test Detects active coronavirus antigen in cats. Supports evaluation of ongoing infection status. Peritonitis Detection Kit Aids in identifying effusive (wet) FIP through fluid analysis. Useful in suspected wet FIP with abdominal or thoracic effusion. Feline 3X (FeLV Ag / FIV Ab / FCoV Ab) Combined screening for retroviruses (FeLV, FIV) and coronavirus antibodies. Provides a broader immunosuppression profile, since co-infections may complicate FIP prognosis. Feline 3DX (FPV Ag / FCoV Ag / Giardia Ag) Detects FCoV antigens along with FPV and Giardia. Useful in differential diagnosis where diarrhea or systemic illness overlaps with FIP. Toxoplasma IgM/IgG Ab Test Screens for toxoplasmosis, a major differential diagnosis for neurological and ocular FIP signs. Helps rule out diseases mimicking dry FIP. Feline Blood Typing Kit Determines blood type before transfusion in severe FIP cases with anemia. Supportive tool for advanced care management. 📌 Note for Veterinarians: For purchase, please contact our sales team or customer service to receive professional consultation and ordering details. 📩 How to Order All rapid test kits listed above are available exclusively to licensed veterinarians and veterinary hospitals. To place an order or request a quotation, please contact our sales team or email our customer service: 📧 service@bioguardlabs.com☎️ Please include your hospital name and contact number in the email so our sales representative can follow up with you directly.
Neoplasia: The Danger of Feline Leukemia Virus
Feline Leukemia Virus (FeLV) remains one of the most consequential retroviruses affecting domestic cats, not only due to its immunosuppressive effects but also its strong association with neoplastic diseases. Among FeLV-positive cats, neoplasia—particularly lymphoma—is a leading cause of mortality. 1. How does FeLV Causes Cancer? FeLV integrates its RNA into the host’s DNA, altering cellular control mechanisms. This can activate oncogenes or disrupt tumor suppressor genes, leading to unchecked cell proliferation. Unlike other viruses, FeLV has a unique ability to directly induce tumor formation, particularly in hematopoietic and lymphoid tissues. 2. Common Types of FeLV-Associated Tumors Lymphoma: The most common neoplasm in FeLV-positive cats, particularly mediastinal, multicentric, and spinal forms. Leukemia: Especially acute lymphoblastic leukemia (ALL), often aggressive and rapidly progressive. Other Neoplasms: Less commonly, FeLV is associated with fibrosarcomas, myeloproliferative disorders, and osteochondromas. 3. Patient Profile and Risk Factors Age: FeLV-related tumors tend to develop in younger cats, often under 5 years of age. Transmission: FeLV spreads through saliva, nasal secretions, and close contact—making multi-cat environments particularly risky. Co-factors: Immunosuppression, co-infections (e.g., FIV), and genetic predisposition may worsen outcomes. 4. Clinical Signs: Generalized lymphadenopathy Dyspnea (especially with mediastinal involvement) Pale mucous membranes, anemia Weight loss, lethargy Neurologic deficits (with spinal lymphoma) GI signs (vomiting, diarrhea, melena) 5. Diagnostic tests: FeLV Testing: Rapid Test, ELISA and PCR testing to confirm infection status. Imaging: Thoracic radiographs, ultrasound, or CT scans for mass detection. Cytology/Histopathology: Fine needle aspirates or biopsies to confirm neoplastic origin. 6. Prevention: Vaccination: FeLV vaccines are effective at reducing infection rates, especially in high-risk populations. Routine Testing: Especially important for kittens, newly adopted cats, and multi-cat households. Environmental Control: Keeping FeLV-positive cats indoors and separated from uninfected cats. FeLV is not only an infectious threat but a potent oncogenic driver. Understanding its role in feline neoplasia underscores the importance of screening, prevention, and early intervention.
Urinary Tract Infections (UTIs) in Dogs: Why my…?

Urinary tract infections (UTIs) are common in dogs and can affect their comfort, health, and behavior. In this article, we will address the common questions that dog owners have regarding their dog urination. Early detection and treatment are essential to prevent complications such as kidney infections or bladder stones. 🔍 What is a UTI in Dogs? A urinary tract infection is typically caused by bacteria (most commonly Escherichia coli) from the rectum, skin, or hair near the entering the urethra and multiplying into the bladder. Less commonly, fungi, virus or other pathogens may be involved. UTIs can affect the lower urinary tract (bladder and urethra) or upper urinary tract (ureters and kidneys). Lower UTIs are more common and less severe, while upper UTIs is very dangerous and can be life-threatening. 🧬 Causes Bacterial contamination (e.g., from feces or environment) Poor hygiene, especially in long-haired or incontinent dogs Urinary retention (not urinating frequently) Underlying health problems: Diabetes mellitus Cushing’s disease Kidney disease Bladder stones or tumors Weakened immune system Congenital abnormalities of the urinary tract 🚨 Clinical Signs Some dogs show clear symptoms, while others may be asymptomatic, especially early on. Pet owners should watch for: Frequent urination (pollakiuria) Straining or pain while urinating Small amount or no urine (dysuria) Blood in urine (hematuria) Cloudy color or foul-smelling urine Accidents in the house (even if previously house-trained) Licking the genital area excessively Fever or lethargy (in severe or kidney-involved infections) Loss of appetite 🩺 Diagnosis A veterinarian will typically perform: Urinalysis – to check for bacteria, white blood cells, pH, crystals Urine culture and sensitivity test – to identify the exact bacteria and the most effective antibiotic Blood tests – to assess kidney function or underlying illness Imaging (X-ray or ultrasound) – if stones, tumors, or structural abnormalities are suspected 💊 Treatment Antibiotics – prescribed based on culture results Pain relief / anti-inflammatories Increased water intake – to flush the urinary system Dietary changes – especially if crystals or stones are present Surgery – in cases of tumors, large stones, or anatomical issues 🔄 Recurring UTIs Recurrent infections may signal underlying problems. Your vet might recommend: Advanced imaging Endoscopy Long-term antibiotic therapy Immune function testing 🛡️ Prevention Tips Ensure clean drinking water at all times (Flowing water is preferred) Encourage regular potty breaks Maintain proper hygiene, especially in long-haired breeds Regular vet checkups, especially for senior dogs Control underlying conditions like diabetes or bladder stones
