Breed-related disease: Ragdolls

In the family of cat breeds, Ragdolls are among the younger siblings. The cats were first developed by breeder Ann Baker who wanted to develop a beautiful cat with a loving, gentle personality, and she started with domestic longhairs of unknown ancestry. Josephine, the foundation cat, was white with Siamese-type markings, and in her genes she carried a seal mitted or black tuxedo pattern. The Ragdolls of today descend from Josephine and her son, Daddy Warbucks, as well as other unknown domestic longhair males. The Cat Fanciers Association began registering Ragdolls in 1993, and they achieved championship status in 2000. Ragdolls are sometimes nicknamed “puppy cats” because of the way they follow their people from room to room, sometimes even taking your privacy from the bathroom. Unlike many cats, Ragdolls are notable for collapsing into the arms of anyone who holds them, even if they are cradled on their back. They love their people, greeting them at the door, following them around the house, and leaping into a lap or snuggling in bed whenever given the chance. They often learn to come when called or to retrieve toys that are thrown for them. All cats have the potential to develop genetic health problems, both pedigreed cats and mixed-breed cats have varying incidences of health problems that may be genetic in nature. Problems that may affect the Ragdoll include the following: Hypertrophic cardiomyopathy : which is a condition in which a portion of the heart becomes thickened without an obvious cause. This results in the heart being less able to pump blood effectively. Symptoms vary from none to feeling tired, leg swelling, and shortness of breath and even sudden death. Blood clots: Ragdolls can be susceptible to blood clots in the arteries. Symptoms include difficulty breathing, loss of appetite, lethargy and depression. Some cats may also faint. Treatment is available, but it is imperative to see a vet as soon as these symptoms appear. Dilated and restricted cardiomyopathy : Dilated cardiomyopathy is caused by the dilation of the heart muscles. Restricted cardiomyopathy is caused by weakened elasticity of the heart muscles. Both can be fatal and need to be detected and treated as soon as possible. Sources: http://www.vetstreet.com/cats/ragdoll#health https://cattime.com/cat-breeds/ragdoll-cats#/slide/1 https://bowwowinsurance.com.au/cats/cat-breeds/ragdoll/
Breed-related disease: Poodle

Poodles are believed to have originated in Germany, though they have been known as French dogs for many years. Their name is derived from the German word pudel, meaning “to splash in water.” Standard poodles are considered the oldest form of this breed. Their history of use as retrievers and water dogs led to the well-known poodle haircut, which was designed to protect joints from cold water temperatures. The breed share a square outline, with a long, elegant neck and a straight back. The tail is docked, but not short, so it can wave gaily. Poodles tend to have a leggy appearance and a long muzzle combined with dropped ears. They move with a springy, lively gait. The coat of the poodle is its crowning glory. Dogs competing in the breed ring must have a specific clip, with areas of extravagant hair coupled with skin tight clipping, these clips actually developed for a reason: they provided thick coat over the joints and chest to keep the dog warm while working in cold water. Intelligent, loving, loyal, and mischievous are four words Poodle enthusiasts commonly use to describe the breed’s personality. Poodles are among the smartest of breeds, but that intelligence can translate into stubbornness. Even so, they can make wonderful therapy dogs. Their empathetic nature and joy in engaging with people make them naturals for visiting with people in nursing homes, hospitals, and schools . Poodles are active dogs, but the smaller dogs need less room and less exercise. Toy and Miniature Poodles are often the companions of people who are less active and can be extremely happy as lap dogs and TV-watching buddies. Just be sure their busy minds have enough to keep them out of mischief. Poodles love to learn and want to please. Trick-training suits their heritage as circus dogs quite well. Teach them to pick up the newspaper, carry a bottle to the recycling bin, and bring your slippers . All dogs have the potential to develop genetic health problems, just as all people have the potential to inherit a particular disease, Poodles are generally considered to be a very sturdy breed with few health issues. Addison’s Disease : Also known as hypoadrenocorticism, this extremely serious condition is caused by an insufficient production of adrenal hormones by the adrenal gland. Most dogs with Addison’s disease vomit, have a poor appetite, and lethargy. Because these signs are vague and can be mistaken for other conditions, it’s easy to miss this disease as a diagnosis until it reaches more advanced stages. Gastric Dilatation-Volvulus : Commonly called bloat, which occurs when gas gets trapped inside a dog’s stomach. The stomach twists as much as 180 degrees, stopping the flow of digestion and causing the gas to build up pressure. It’s an incredibly painful disorder with a 20% mortality rate even with surgical intervention. Deep-chested breeds like the standard Poodle or Great Dane are especially prone to experiencing bloat. Hip Dysplasia : A dog’s hip operates on a ball-and-socket joint. When the socket is over- or under-developed, or when the ligaments holding the two together are weak, the ball can become dislodged. This constant dislocation and relocation will wear down the joint’s integrity and cause hip dysplasia. Hypothyroidism: Hypothyroidism is caused by an under active thyroid gland. It’s thought to be responsible for conditions such as epilepsy, hair loss, obesity, lethargy, hyperpigmentation, pyoderma and other skin conditions. Epileptic : Epileptic seizures in dogs are as shocking for canines as they are for humans. Dogs will often seem confused or panicked about what’s happening, and the sight of your dog seizing can be terrifying to watch. When this happens, you need to remain calm and focus on helping your dog. https://dogtime.com/dog-breeds/poodle#/slide/1 https://en.wikipedia.org/wiki/Poodle
Feline Calicivirus Review: Biology, Risks, and Care

Table of Contents 1. Introduction: Feline Calicivirus in Veterinary Practice 1.1 Background Feline Calicivirus (FCV) remains one of the most clinically significant viral pathogens affecting domestic cats, particularly as a leading cause of upper respiratory tract infections (URTIs). Its impact is amplified by the virus’s intrinsic biological properties: FCV exhibits high genetic variability, a remarkable ability to persist in chronically infected carriers, and environmental stability that enables sustained circulation within shelters, colonies, and multi-cat households. These features contribute not only to recurrent outbreaks but also to efficient viral maintenance within feline populations worldwide. From a virological standpoint, FCV is a small, non-enveloped, icosahedral virus, measuring approximately 30–40 nm in diameter and harboring a single-stranded positive-sense RNA genome of roughly 7.7 kb. Its use of junctional adhesion molecule-1 (JAM-1) as a cellular receptor facilitates viral entry and dissemination. Infection typically begins following exposure through the nasal, oral, or conjunctival routes, with the oropharynx serving as the primary site of replication. Viral amplification here drives epithelial necrosis, leading to the characteristic oral ulcerations most commonly identified along the margins of the tongue. Given the combination of widespread prevalence, substantial morbidity, and occasional highly virulent systemic outbreaks, a consolidated review of FCV’s structure, replication biology, epidemiology and clinical behavior remains essential for contemporary veterinary practice. 1.2 Objectives This article aims to synthesize current evidence from molecular virology, clinical epidemiology and field management to guide veterinary professionals, shelter medicine practitioners and cattery managers. Summarize evidence-based findings on FCV structure, replication and pathogenesis.A molecular understanding of FCV underpins rational approaches to diagnosis, prevention and therapeutic intervention. Particular focus is placed on capsid architecture, genomic organization, antigenic variability and the early host–virus interactions that shape clinical outcomes. Provide veterinarians and cattery managers with practical prevention and care strategies.In high-density environments, controlling FCV transmission requires a layered approach that combines:• vaccination programs,• minimizing population stress and overcrowding,• rigorous hygiene and environmental disinfection, and• timely clinical management of affected cats. Although vaccination remains central to disease mitigation, it typically does not prevent infection or viral shedding, and breakthrough infections continue to occur. Effective clinical management therefore relies heavily on supportive measures such as intravenous fluid therapy for dehydration, nonsteroidal anti-inflammatory drugs for pyrexia and oral pain, and targeted antibiotics for secondary bacterial complications. Highlight key clinical risks, including oral ulceration, FCV-associated lameness and virulent systemic disease (VSD). Oral Ulceration:A hallmark of classical FCV infection, especially in kittens, appearing after an incubation period of 2–10 days. It is frequently accompanied by sneezing and serous nasal discharge. FCV-Associated Lameness:Characterized by acute synovitis with joint effusion and synovial membrane thickening. This syndrome may arise days to weeks after respiratory signs or following vaccination. • Virulent Systemic Disease (VSD):A rare, highly pathogenic phenotype with reported mortality rates up to 67 percent. VSD is marked by systemic inflammatory response syndrome, disseminated coagulopathy and multi-organ failure. Clinically, affected cats exhibit severe URTI signs followed by cutaneous ulcerations, alopecia of the extremities, broncho-interstitial pneumonia and necrosis of major organs including the liver, spleen and pancreas. Management requires intensive supportive care, often incorporating corticosteroids and interferon. 2. Viral Structure and Molecular Biology Feline Calicivirus (FCV) belongs to the Caliciviridae family, a group of small, non-enveloped RNA viruses characterized by compact genomes and efficient replication strategies. The structural and molecular features of FCV underpin its clinical behavior, including its ability to persist, diversify, and evade immune surveillance in feline populations. 2.1 Virion Architecture FCV is a non-enveloped, icosahedral virus measuring 30–40 nm in diameter. Its capsid is considered “naked”, reflecting the absence of a lipid envelope. The virion is composed of a single capsid protein, with a precursor mass of 65–66 kDa, which is later processed into the major structural protein VP1. This protein forms the characteristic icosahedral shell that encases the viral RNA genome. 2.2 Genome Organization The genome of FCV consists of a single-stranded, positive-sense RNA molecule of approximately 7.7 kb, organized into three Open Reading Frames (ORFs): ORF1 – Non-structural Polyprotein Encodes a 200 kDa polyprotein that undergoes proteolytic cleavage to produce six mature non-structural proteins, essential for RNA replication and virion assembly. ORF2 – Capsid Precursor (preVP1) Encodes a 73 kDa capsid precursor (preVP1).• Undergoes rapid cleavage during maturation to yield the 60 kDa VP1 capsid protein.• Subdivided into regions A–F; among these, the E-region determines antigenicity and contributes to formation of the P2 subdomain, a key external protrusion involved in receptor interaction and immune recognition. ORF3 – Minor Structural Protein VP2 Encodes VP2, a 12 kDa protein (106 amino acids) that, although less abundant, is essential for producing infectious virions and supports VP1 stability during assembly. https://share.google/unsumbG6JXsez5x1R 2.3 Replication Cycle FCV replication proceeds through the synthesis of: A 7.7 kb genomic RNA (positive-sense)• A 2.4 kb subgenomic RNA, which serves as the template for capsid protein translation Viral entry is mediated by junctional adhesion molecule-1 (JAM-1), identified as the functional receptor during in vitro studies. Once inside the host cell, FCV induces a characteristic cytopathic effect (CPE)—notably cell rounding and membrane blebbing. A central mechanism of viral dominance is the shut-off of host protein synthesis, accomplished through cleavage of eIF4G, a critical eukaryotic initiation factor. This redirection enables preferential translation of viral RNA and efficient progeny production. 3. Epidemiology 3.1 Global Distribution Feline Calicivirus (FCV) was first isolated from the gastrointestinal tract of cats in New Zealand (Fastier, 1957). Since that initial discovery, FCV has been recognized as a globally widespread pathogen, circulating in domestic and free-roaming feline populations across continents. Its prevalence is particularly high in high-density environments, such as multi-cat households, shelters, and breeding colonies. Studies consistently report 25–40 percent infection rates among cats in colonies and shelters (Wardley et al. 1974, Bannasch & Foley 2005). The combination of environmental persistence, antigenic diversity, and efficient cat-to-cat transmission ensures FCV remains an endemic viral pathogen in most feline communities worldwide. 3.2 Transmission and Persistence FCV transmission occurs predominantly through oral, nasal, or conjunctival exposure. Direct contact with secretions, as well as indirect exposure through contaminated fomites, facilitates rapid spread—particularly in
Canine blood-typing
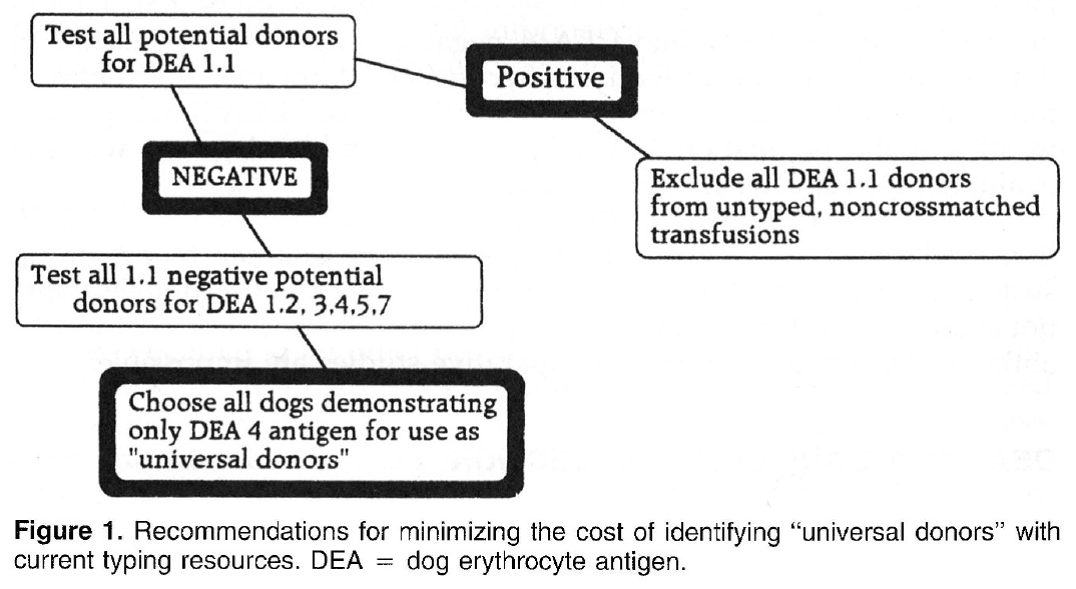
Canine blood-typing Andy Pachikerl, Ph.D Introduction: Dog erythrocyte antigens are responsible for initiating approximately 70% to 80% of immune-mediated transfusion reactions in the dog. As with other species, the red blood cell antigens found in the dog have various immunogenicities. In health, these antigens are participants in cell recognition-self versus nonself. In disease, they may serve as antigens for antibody or markers in disease occurrence. Little is known about their biochemical properties. Currently their description is reliant on polyclonal antibody serology. This reliance has limited the progress of transfusion practice in the dog. Historically, the study of canine blood groups and their importance in transfusion began in the 1600s through a physician, Richard Lower. He is credited with the first canine-to-canine transfusion. The efforts of doctors Lower and Denis in heterologous transfusion using lamb, dog, and human subjects introduced the basic transfusion premise “like transfuses like.” In 1910, Von Dungem and Hirszfeld documented the presence of four hemolysins and agglutinins based on canine alloimmunization (Swisher & Young, 1961). Further work by Ottenburg, Kaliski, and Friedman in 1913 confirmed these findings. From 1937 to 1949, Wright, Whipple, and Eyquem further defined the presence of six canine blood groups (Colling & Saison, 1980). However, not until 1961 was the importance of these antigens in transfusion and disease investigated by Swisher, Young, and Trabold (Swisher, et al., 1962). To date, the work submitted by Swisher and Young remains the most current published information of the importance of canine blood groups in transfusion (Swisher, 1954; Swisher, et al., 1962; Young, et al., 1951). Additional blood groups have been identified by Rubenstein (1968), Suzuki/5 Colling and Saison (Colling & Saison, 1980; Colling & Saison, 1980), and Symons and BelLl9 (Symons & Bell, 1992). Of this latter group, only the antigen first noted by Rubenstein (Colling & Saison, 1980) was evaluated in regard to transfusion significance by Bull (Bull, 1976). The importance of canine blood groups in veterinary transfusion medicine is based on three factors: the incidence of the antigen in the dog population, the incidence of naturally occurring antibody within the population, and the effect of the antibody against the antigen in vivo. Current blood typing schemes identify six erythrocyte antigens with possible importance. The dog erythrocyte system Blood groups are defined by glycolipids and glycoproteins on the surface of the red blood cell membrane. Current blood typing schemes identify six dog erythrocyte antigens (DEAs): 1.1, 1.2, 3, 4, 5, and 7 (Table 1). Blood groups are independently inherited. Simple Mendelian laws of dominance govern their inheritance. These antigens are defined by using polyclonal antibodies generated through canine alloimmunization. Polyclonal antibody recognition may be dependent on multiple recognition sites to define the “antigens” currently accepted. Biochemically, little is known about the DEA system. Table 1. Dog erythrocyte antigens established as international standards: classification, occurrence, and significance This blood group system has been defined with multiple alleles. They include the antigens 1.1, 1.2, 1.3, and a null type. An individual dog may show only one of the four phenotypes. Family studies suggest a Mendelian type of autosomal dominance. Table 2 describes the current phenotypic and genotypic information on this blood group system. 1.1- and 1.2-positive dogs have been studied for transfusion significance. Naturally occurring antibody to these alleles has not yet been found. Therefore, first-time transfusion reactions do not occur. However, if a negative dog is exposed to 1.1- positive erythrocytes, a strong hemolysin can result. On second exposure, an immune-mediated hemolytic transfusion reaction results causing removal of transfused cells in less than 12 hours. Hemoglobinuria and hyperbilirubinemia frequently occur. In addition to uncross matched, untyped transfusion, pregnancy can cause production of antibody against DEA 1.1 25% of the time. For these reasons 1.1-positive dogs are excluded as transfusion donors. 1.2-positive dogs can cause a problem as both the transfusion donor and recipient. A previously sensitized negative type dog undergoes permanent red blood cell removal and loss 12 to 24 hours after the administration of 1.2-positive red blood cells. Thus, 1.2-positive dogs are poor erythrocyte donors. If a 1.2-positive dog is sensitized with DEA 1.1 red blood cells, it will produce a potent anti-DEA 1.1 antibody. Administration of DEA 1.1 red blood cells to a sensitized 1.2 dog results in an immediate hemolytic transfusion reaction. Therefore, 1.2-positive dogs are at risk after sensitization for immediate transfusion. 1.3-positive dogs have not been evaluated for transfusion significance. Future study is limited because of the unavailability of typing sera for DEA 1.3. DEA 7 This red blood cell antigen is the most controversial among the six antigens discussed. Published reports of naturally occurring antibody to this antigen suggest that this antibody has a natural prevalence as high as 50% in DEA 7-negative dogs. Recent reports by Giger fail to support the presence of naturally occurring anti-DEA 7. Observations by the author suggest that naturally occurring antibody does exist in 20% to 50% of all DEA 7-negative dogs. However, the naturally occurring antibody is quite weak, rarely producing a titter greater than 1:8. In the presence of naturally occurring antibody, as in the cat, immunemediated transfusion reaction can occur during a first transfusion. Sensitized DEA 7-negative dogs, when transfused with DEA 7-positive erythrocytes show a delayed transfusion reaction. Hemolysis does not occur; however, an irreversible sequestration and loss of red blood cells occurs in 72 hours. This type of delayed transfusion reaction is only significant if the regenerative ability of the transfusion recipient is compromised. Because of the presence of naturally occurring antibody in the DEA 7-negative population and because of the delayed loss of erythrocytes in sensitized dogs, DEA 7-positive dogs are not recommended as donors. DEA3 This antigen has not been considered significant because of its low incidence in the dog population of the United States. However, recent evaluation of DEA type by breed suggests that it may be more important. Only 6% of the general population has DEA 3-positive cells. Yet 23% of the Greyhounds typed from 1990 to
Breed-related disease: Sphynx cat

The Sphynx cat is a breed of cat known for its lack of coat (fur). Hairlessness in cats is a naturally occurring genetic mutation; however, the Sphynx cat, as a breed, was developed through selective breeding. The Sphynx first appeared as a natural mutation in Canada in 1966. The first hairless male, Prune, was mated back to his mother, and some of their hairless kittens were exported to Europe, where they acquired the breed’s name. Despite appearances they’re not completely naked and their skin has the texture of a peach – Sphynx should not be described as bald! They’re warm to the touch too! It really is difficult to judge or appreciate Sphynx just from photographs. What wins people over, beyond the appeal of the unusual, is their larger-than-life characters. It is possibly one of the most affectionate, sociable and intelligent cats in the world, they adore human attention and enjoy cuddles and games. They are outgoing, mischievous, people-orientated and loves attention. These cats often greet their owners when they come home and are very talkative. They are highly intelligent, playful and cuddly. They like to sleep with their owners – under the covers. Their body temperature is a degree or two above the average for normal cats and they have voracious appetites to compensate for the heat loss. With little protection against the elements, these cats cannot be left out in the cold, they don’t like to sit on cold surfaces and they do appreciate central heating! Those that do go outside in the sun may need sun protection on pale skin. We know that because you care so much about your cat, you want to take great care of her. That is why we have summarized the health concerns we will be discussing with you over the life of your Sphynx. By knowing about the health concerns common among Sphynxes, we can help you tailor an individual preventive health plan and hopefully prevent some predictable risks in your pet. Heart Disease: the sphinx is prone to cardiomyopathy which is the medical term for heart muscle disease, either a primary inherited condition or secondary to other diseases that damage the heart. The most common form, called hypertrophic cardiomyopathy, or HCM, is a thickening of the heart muscle often caused by an overactive thyroid gland. Blood Type: Just like people, individual cats have different blood types. Most domestic cats have type A blood, but purebred cats, like your Sphynx often have a different blood type, usually type B or very rarely, type AB. Determining your cat’s blood type is essential before starting a transfusion, so knowing your cat’s type ahead of time can save crucial minutes. For more information regarding the blood typing please visit following link: https://www.bioguard.com.tw/en/project/feline-blood-typing-kit/ Alopecia: If you thought you were getting out of grooming chores by adopting a Sphynx cat, think again! Your sphynx Cat’s skin will build up a greasy grunge if left unbathed—and don’t forget the ears! The insides of the ears will get waxy and need to be cleaned periodically as well. Urticaria Pigmentosa: There is a long list of diseases that can make your cat itch and break out in little red bumps. Allergies to food or to pollen, parasites like fleas or mites, fungal or bacterial infections, and even certain types of autoimmune diseases can all cause these general symptoms. But for your Sphynx, add urticaria pigmentosa to the list. The exact pathology of this itchy skin disease has not yet been fully discovered, but it appears to be passed on genetically, and is fairly common in some family bloodlines. With so many possibilities as the cause for apparently identical skin irritations, diagnostic testing is essential in order to narrow down treatment options. Sources: https://www.yourcat.co.uk/types-of-cats/sphynx-cat-breed-information/ https://chesapeakevetclinic.com/client-resources/breed-info/sphynx/ photo credit: https://www.freepik.com/premium-photo/hairless-canadian-sphynx-cat-with-bow-tie-isolated_6884985.htm
Case study: Cerebral toxoplasmosis in a cat with feline leukemia and feline infectious peritonitis viral infections
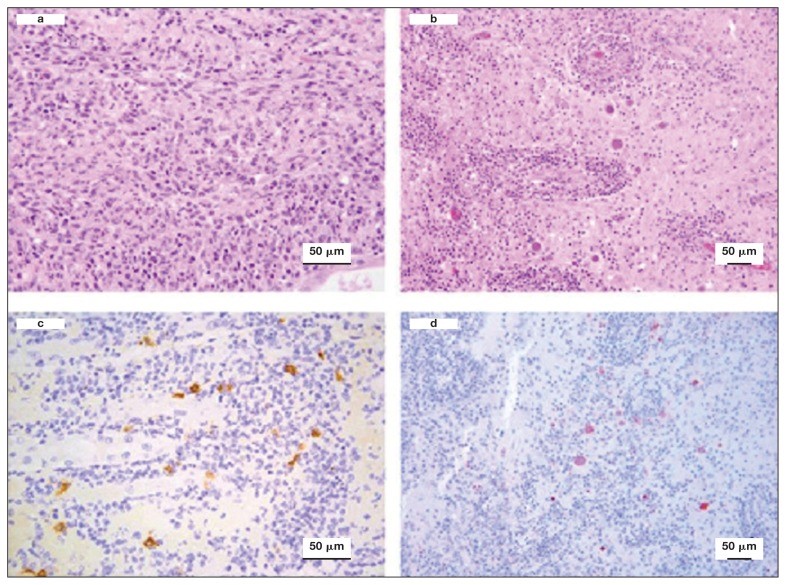
Case study: Cerebral toxoplasmosis in a cat with feline leukemia and feline infectious peritonitis viral infections Robert Lo, Ph.D, D.V.M A diarrheic young cat died because of severe multifocal meningoencephalitis caused by Toxoplasma gondii. Protozoan cysts and tachyzoites in the brain were confirmed by immunohistochemical staining. Coinfection of feline leukemia virus (FeLV) and feline infectious peritonitis (FIP) might be the possible contributors to the clinical, fatal outcome. Original paper: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6049326/ Histological lesions and immunohistochemistry (IHC) of the brain and kidney of a 6-month-old cat that was diagnosed with cerebral toxoplasmosis following postmortem examination. a — Kidney showing chronic pyogranulomatous nephritis. Hematoxylin and eosin (H&E), 40×. b — Brain showing perivascular cuffing of lymphocytes and plasma cells with multifocal vasculitis. Many oval protozoan cysts having a thin wall and containing basophilic bradyzoites were seen near to the vascular structures. H&E, 20×. c — Kidney stained by IHC with feline coronavirus antibodies showing multifocal positive reaction in the cytoplasm of macrophages, 40×. d — Brain stained by IHC with anti-Toxoplasma gondii antibodies and showing several positively stained protozoan cysts and tachyzoites, 20×.
The Shiba Inu

John K. Rosembert The Shiba Inu dog breed was originally bred to flush birds and small game and was occasionally used to hunt wild boar. It is the smallest of the six original and distinct spitz breeds of dog native to Japan. the Shiba Inu dog almost died out entirely in the Second World War but the small number of dogs who had survived bombing raids and a distemper epidemic were bred to save the breed. It is a small, compact dog, their head is in proportion with the body with a round muzzle that has a moderate stop and tapers slightly toward the nose. The tight lips and the nose are black. The teeth meet in a scissors bite. The deep-set eyes are triangular in shape and dark in color. The well-bred Shiba Inu is good-natured, alert, and bold. He is strong-willed and confident and often has his own ideas about things. He is loyal and affectionate with his family, though tends to be suspicious of strangers. The Shiba Inu doesn’t share well. He tends to guard, sometimes aggressively, his food, toys, or territory. And he doesn’t always get along with other dogs, especially if he’s intact. He won’t hesitate to chase small animals that he considers prey. This is a smart breed, but training a Shiba Inu isn’t like training a Golden Retriever. While a Golden is delighted to come when called, the Shiba Inu will come when he feels like it — or not. He’s been described as stubborn, but freethinking is probably a more positive way to characterize him. Below we will discuss the most common health problems that Shiba Inu may encounter during his lifetime. Eye Problems: Not many things have as dramatic an impact on your dog’s quality of life as the proper functioning of his eyes. Unfortunately, Shiba Inus can inherit or develop several different eye conditions, some of which may cause blindness if not treated right away, and most of which can be extremely painful! We will evaluate his eyes at every examination to look for any signs of concern. Ex: Glaucoma, Cataracts, Distichiasis, Eyeballs. Pyometra: If a female Shiba Inu hasn’t been spayed, then they can experience pyometra during their heat cycle. It occurs when the growth of cells in the uterus is at its highest production rate (this happens during their heat cycle), in which bacteria can migrate into the area and cause a life-threatening infection. While this condition can occur to all-female canines, it seems to be a bit more prominent with Shibas, which only furthers the reason they should be spayed. Heart Disease: Some breeds like your Shiba can be born with a variety of heart defects. Most of these affect the structure of the heart’s dividing wall or the vessels. They can also cause problems with the electrical signals that control the heartbeat or with heart valve function. Allergies: In humans, an allergy to pollen, mold, or dust makes people sneeze and their eyes itch. In dogs, rather than sneeze, allergies make their skin itchy. We call this skin allergy “atopy”, and Shibas often have it. Commonly, the feet, belly, folds of the skin, and ears are most affected. Symptoms typically start between the ages of one and three and can get worse every year. Licking the paws, rubbing the face, and frequent ear infections are the most common signs. The good news is that there are many treatment options available for this condition. Seizures: Seizures in dogs aren’t exactly similar to what humans are used to. They can take shape as the Shiba Inu running around ceaselessly, hiding in corners in complete confusion, barking at nothing, and freezing up. While seizures are usually not life -threatening for dogs, they can also be indicative of a more serious problem. https://dogtime.com/dog-breeds/shiba-inu#/slide/1 https://animalhealthcenternh.com/client-resources/breed-info/shiba-inu/ https://canna-pet.com/shiba-inu-health-problems-issues/ Photo credit: https://thehappypuppysite.com/shiba-inu-temperament/ https://en.wikipedia.org/wiki/Shiba_Inu
Breed-related disease: Scottish Fold

John K. Rosembert The Scottish Fold is a medium-size cat with a rounded head and big round eyes, although he is known for his standout feature: ears that fold forward, giving him the appearance of a furry owl. His coat, which comes in many colors and patterns, can be short or long. The long-haired variety is known as the Highland Fold. Drop-eared cats such as the Scottish Fold are not as unusual as they might seem. Spontaneous genetic mutations, such as curly coats or folded ears, occur in cats on a fairly regular basis, and the Scottish Fold is the result of such a mutation. All of today’s Folds descend from a Scottish fold-eared cat named Susie who was found by a shepherd in 1961. Scottish Fold kittens are born with what look like normal ears. The ears soon begin to bend forward, usually two to four weeks after birth. By the time he is three months old, the typical Scottish Fold has a distinctly owlish look, the tightly folded ears do not appear to be any more prone to infections than those of cats with upright ears. However, this unique ear shape is caused by an underlying defect in the formation of cartilage, which would normally retain the ears in a normal shape. This inherited cartilage defect (also known as Scottish Fold disease, or Osteochondrodysplasia) causes other deformities throughout the body and is a dominant trait, meaning all kittens in the litter will be affected. The disease is evident on x-rays of cats from as young as 7 weeks of age. Serious abnormalities in joints and bone growth lead to arthritis (painful, swollen joints), short, abnormally thick and inflexible tails, spinal abnormalities and short, stiff legs. The welfare impacts are severe in terms of pain and inability to perform natural behaviors, as these cats can be lame, walk with an abnormal gait, can be reluctant to engage in normal movements such as walking or jumping, and can even become completely crippled. That’s why breeding the Scottish Fold is banned in some country. The Scottish Fold is a great family pet. They are very loyal to their family. They are not a shy breed that would hide around the house; they prefer always being around and following owners from room to room. Scottish Folds are very intelligent and inquisitive. They learn to open cabinets, play fetch, sit up, and some like to eat & drink with their paws. Most love to drink from running water! Most Scottish Folds also “sit up” like a prairie dog when they hear something to get a look around. Below we summarized some of the most common health issues of Scottish Fold cat in order to help you prevent some predictable risks in your pet. Dental disease is one of the most common chronic problems in pets who don’t have their teeth brushed regularly. Unfortunately, most cats don’t take very good care of their own teeth, and this probably includes your Fold. Polycystic kidney disease (PKD) is caused by a defective gene. The disease was first recognized in Persians, and is seen occasionally in other breeds, including Shorthaired Scottish Folds. Affected kittens are born with miniscule cysts inside the kidneys and sometimes the liver that slowly enlarge over time, eventually destroying the affected organ. FLUTD Feline lower urinary tract disease (FLUTD) is not a specific disease, but rather is the term used to describe conditions that can affect the urinary bladder and/or urethra (the lower urinary tract) of cats. This situation is very common in Scottish Fold. Sources https://canalclinic.com/client-resources/breed-info/scottish-fold-shorthair/ Photo credit https://scottishfoldcats.net/scottish-fold-munchkin-cat-price/ https://commons.wikimedia.org/wiki/File:Scottish_fold_cat.jpg
The Feline Herpesvirus: An Overview
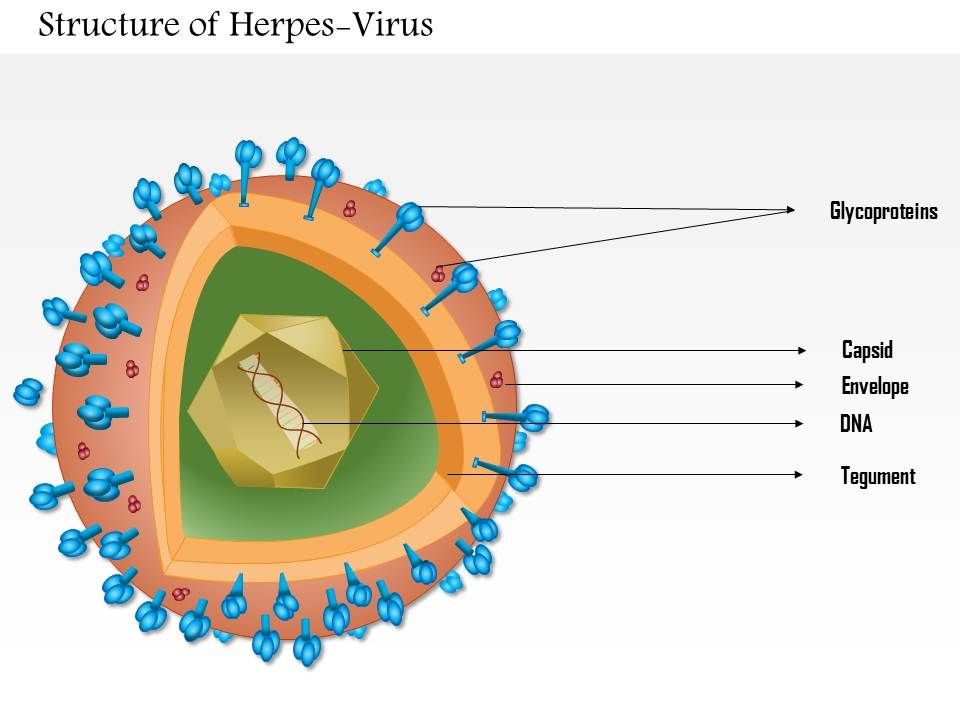
The Feline Herpesvirus: An Overview Maigan Espinili Maruquin The feline herpesvirus infection is common and recurring ocular disease is common (Stiles 2000). It is the most studied infectious cause of ocular surface disease in cats (Andrew 2001). Developing latent infections may recrudesce at later points in life of an infected cat (Stiles 2000). I. Structure and Replication Fig. 01. Structure of Herpes Virus. https://www.slideteam.net/0814-herpes-virus-medical-images-for-powerpoint.html The feline herpesvirus (FHV 1) causes feline viral rhinotracheitis (FVR) (Gaskell and Wardley 1978, Gaskell, Dawson et al. 2007, Henzel, Brum et al. 2012). This virus is double-stranded DNA with a glycoprotein-lipid envelope and is a member of the Varicellovirus genus in the Alphaherpesvirinae subfamily (Gaskell, Dawson et al. 2007). This virus was also found out to be relatively fragile in the external environment and is highly susceptible to the effects of common disinfectants (Scott 1980, Eleraky, Potgieter et al. 2002, Gaskell, Dawson et al. 2007). The FHV- 1 has short replication cycle, rapid cell-to-cell spread, has tendency to induce cell lysis, and displays persistence in sensory ganglia of their host (Gould 2011). It replicates in epithelial cells of both the conjunctiva and upper respiratory tract, and in neurons. The neuronal infection can lead to a lifelong latency after the primary infection (Thiry, Addie et al. 2009). For 18 hours, it can survive in damp environment, less in dry conditions and is also recorded to be relatively unstable as an aerosol (Povey and Johnson 1970, Donaldson and Ferris 1976, Stiles 2000, Gaskell, Dawson et al. 2007, Gould 2011). II. Infection and Epidemiology There are only three main genotype groups recognized for FHV-1 strains with very little genomic variations (Gould 2011). The virus sheds in ocular, nasal, and oral secretions with large transmission by direct contact with an infected cat. Although one of the most important sources of virus are the acutely infected cats, susceptible cats may also be infected by latently infected carrier cats (Gaskell and Povey 1982, Gaskell, Dawson et al. 2007). On the other hand, the environment may not be a primary source of transmission but catteries may cause indirect transmission through contaminated housing, feeding and cleaning utensils, and personnel (Gaskell, Dawson et al. 2007, Thiry, Addie et al. 2009). Latently infected cats may also transmit FHV to their kittens due to the parturition and lactation inducing stress that may lead to viral reactivation and shedding, making kittens susceptible to the virus, depending on the level of maternally derived antibodies (MDA) they possess. High levels of MDA protects kittens against the disease and may develop subclinical infection leading to latency while insufficient MDA may lead to clinical signs (Gaskell and Povey 1982, Thiry, Addie et al. 2009). Recovered cats become latently infected carriers and reactivation happens particularly after periods of stress (Gaskell, Dawson et al. 2007). However, it doesn’t shed immediately after the stress. It undergoes lag phase of 4–11 days, precedes the shedding from 1–13 days (Gaskell and Povey 1973, Gaskell and Povey 1977, Gaskell, Dawson et al. 2007). Further, risk factors associated with FeHV-1 shedding includes contact with other cats, the presence of upper respiratory disease, younger cats, poor hygiene, and larger households (Sykes, Anderson et al. 1999, Binns, Dawson et al. 2000, Helps, Lait et al. 2005, Gaskell, Dawson et al. 2007). III. Pathogenesis Infection routes include nasal, oral, and conjunctival mucous membranes and is primarily shed in secretions for 1–3 weeks following infection (Andrew 2001, Gaskell, Dawson et al. 2007). In pregnant queens, vaginitis was caused by intravaginal instillation virus and causes congenitally infected kittens while intravenous inoculation leads to transplacental infection and abortion (Bittle and Peckham 1971, Hoover and Griesemer 1971, Gaskell, Dawson et al. 2007). After 1 to 2 exposure of naive animals to FHV-1, the viral replication happens and epithelial cell necrosis occur in the nasal turbinates, nasopharynx and conjunctival mucosa (Gaskell & Dawson 1998). Lytic infection of the nasal epithelium with spread to the conjunctivas, pharynx, trachea, bronchi and bronchioles occurs and lesions characterized by multifocal epithelial necrosis with neutrophil infiltration and inflammation are also observed. Moreover, neonates or hypothermic kittens display transient viraemia associated with mononuclear cells (Gaskell, Dawson et al. 2007, Thiry, Addie et al. 2009). It has been recorded that almost all infected cats become lifelong carriers. During the latency period, virus was spread along the sensory nerves and neurons with viral genome doesn’t replicate. Whereas, reactivating stressors include lactation and moving into a new environment (Gaskell and Povey 1977, Gaskell and Povey 1982, Pedersen, Sato et al. 2004, Thiry, Addie et al. 2009). Lesions may be developed upon viral reactivation in adult cats and ‘recrudescence’ disease may also be a consequence (Thiry, Addie et al. 2009). As high as 70% mortality rates was reported for infected kittens (Povey 1990). Although MDA may persist for 2 to 10 weeks, this may not protect cats from subclinical infection (Gaskell & Dawson 1998)(Andrew 2001). IV. Clinical Signs Generally, FHV- infected cats display acute upper respiratory and ocular disease with usually 2 to 6 days incubation period, or may be longer (Gaskell and Povey 1979, Stiles 2000, Gaskell, Dawson et al. 2007) with depression, fever, lethargy, inappetence, pyrexia, sneezing, coughing, nasal discharge, and conjunctivitis with ocular discharge depending on the viral exposure and individual susceptibility (Hoover, Rohovsky et al. 1970, Crandell 1973, Stiles 2000, Gaskell, Dawson et al. 2007, Thiry, Addie et al. 2009) (Gaskell R.M., Dawson S, 1994). Also, excessive salivation with drooling may also be observed during the initial clinical signs of the disease (Gaskell, Dawson et al. 2007).Once the virus reaches the lungs, pneumonia may kill the infected kittens (Stiles 2000, Thiry, Addie et al. 2009) (Gaskell R, et al. 2006). The primary FHV- 1 infection with secondary bacterial infection leads in conjunctivitis sometimes with severe hyperemia and chemosis. The conjunctivitis is manifested as hyperaemia or redness with serous discharge, progressing to mucopurulent ocular discharge whereas, chemosis is swelling or oedema of the conjunctiva which may occur to
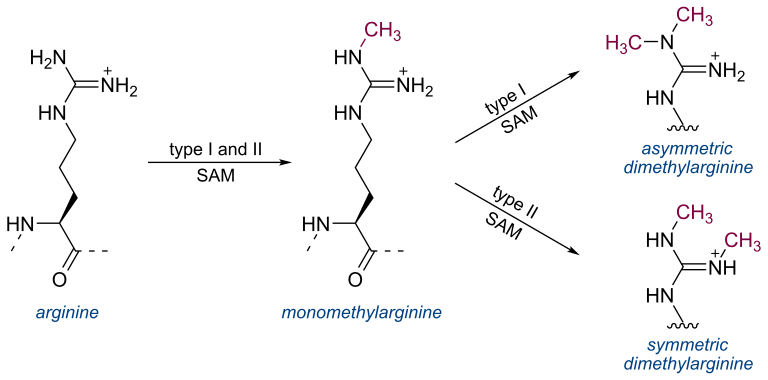
Symmetric dimethylarginine (SDMA)
Symmetric dimethylarginine (SDMA) Andy Pachikerl, Ph.D Introduction For over a millennium and a few centuries, urinalysis has given leads to medical diagnoses. It was until the repetitive use of clinical chemistry approximately 50 – 60 years that these data of renal biomarkers became commonplace in human and veterinary medicine. From here onwards, both an improved understanding of the renal system and ability to diagnose renal disease was updated. In the past, renal biomarkers have focused on kidney function testing, and this is the basis for current conventional test in blood (serum creatine [sCr], urea or urea nitrogen [UN] as endogenous indicators of glomerular filtration rate [GFR]). Recently, we are becoming more aware of the need to identify renal disease at an early stage when therapeutic options are most effective. Both sCr and UN both play vital role in diagnosis of kidney disease, their limitations create poor confidence for their use as early indicators for disease. New markers of renal function try to overcome these limitations. Additionally, there are now many urinary markers that can detect kidney damage and help localize that damage to the compartment of the kidney that is affected. Endogenous markers of GFR Creatine The most common endogenous marker for estimating GFR is serum creatinine and its metabolism. Measurement and diagnostic significance in dogs have previously been reviewed (Braun, et al., 2003). Recent reviews, however, suggested factors that can either enhance or limit the clinical use of sCr to optimize diagnostician and clinical pathologist to interpret the data of this conventional test. Particularly, accurate interpretation of published data, population-specific reference intervals, trending of sCr and consideration of muscle mass influence and analytic variability are all needed to best interpret sCr in dogs and cats. Of note, although creatinine is referred to as sCr throughout this manuscript, creatinine is also commonly measured in plasma. Nephron mass vs nephron function. It is generally accepted that 75% of nephron mass must be lost before sCr increases above the reference limit (Braun, et al., 2003). The original source for this statement likely originates from partial nephrectomy studies in dogs. However, it is often mistaken for 75% loss of renal function vs mass. In partial nephrectomy studies, ¾ loss of renal mass related to about 50–60% or 35–45% reduction in renal function based on inulin clearance one month or 13 months post-surgery, respectively (Brown, et al., 1990; Bovee, et al., 1979). The much lower decrease in function as compared with the percentage of nephron loss is due to compensatory changes in remaining nephrons (ie, compensatory renal hypertrophy) (Brown, et al., 1990; Bricker, et al., 1964). Furthermore, using an age- and breed-specific reference limit (sCr ≥ 106 mmol/L or 1.2 mg/dL) along with frequent monitoring, adolescent dogs with rapidly progressive kidney disease due to X-linked hereditary nephropathy (XLHN) demonstrated increased sCr after GFR had decreased an average of 48% (range 39–68%).8 Based on these studies, sCr can be more sensitive for detecting decreased renal function than has been historically assumed. Value of population-specific reference intervals. While sCr is not as poorly sensitive as generally believed, its inability to regularly detect < 50% decline in kidney function at least partly stems from reference intervals that are overly wide for patients with low baseline sCr. Since current methodologies are highly specific for creatinine, the wide reference intervals largely stem from biologic differences in sCr among individuals. Serum creatinine has relatively high individuality in dogs and cats (Baral, et al., 2014; Ruaux, et al., 2011), meaning that variability between individuals is much higher than the variability observed within a single animal. Serum creatinine is influenced by age (Rosset, et al., 2012; Rørtveit, et al., 2015) and particularly by breed in dogs (Misbach, et al., 2014; Zaldívar-López, et al., 2011) and, to a lesser extent, in cats.20 It might also be influenced by sex and the veterinary clinic evaluating the patient.21 Therefore, sCr would benefit from age- and breed-specific reference intervals, ideally (although not practically) for every individual instrument and laboratory. Trending of serum creatinine. Small increases in sCr even within the reference interval can reflect significant decreases in GFR in an individual patient8, particularly since variation in sCr within an individual healthy dog or cat is minimal over weeks to months and even years (Braun, et al., 2003; Baral, et al., 2014; Ruaux, et al., 2011). In fact, the critical difference or reference change value for detecting a significant increase or decrease in sCr is only 23–27 lM/L (0.3 mg/dL) in clinically healthy dogs10, and 17% (corresponding to similar absolute values as in dogs) in clinically healthy cats (Braun, et al., 2003). Thus, the sensitivity of sCr for detecting early kidney disease can be improved by evaluating serial fasted sCr measurements in an individual animal (trending) to look for increases that likely reflect worsening renal function. This concept of detecting small but clinically relevant increases in sCr is actively being adopted in cases of acute kidney injury (AKI), illustrated by the International Renal Interest Society (IRIS) Grading of AKI. In this grading scheme, an increase in sCr ≥ 26 lmol/L (0.3 mg/dL) within a 48- hour period is a criterion for identifying Grade I and Grade II AKI (www.IRIS-Kidney.com). Furthermore, in adolescent dogs with XLHN, trending of sCr detected an average of 27% (range 5–49%) decrease in GFR (Nabity, et al., 2015). Despite heightened awareness of small, but clinically relevant increases in sCr over a short time frame, more recognition is needed with slowly progressive CKD, in which small increases might occur over many months or years. Analytic challenges. Finally, sCr is plagued by inconsistencies in its measurement between instruments and laboratories, which can result in markedly different results. While most reference laboratory instruments have excellent precision and provide results of similar magnitude among instruments (Ulleberg, et al., 2011), recent studies illustrate the high imprecision and bias possible with some instruments and among different laboratories (Ulleberg, et al., 2011; Braun, et al., 2008). In normal to mildly azotaemia samples,
