The Danger of Antimicrobial Resistance (AMR) in Animals
Learn how antimicrobial resistance in animals develops, its impact on animal and human health, and the critical role of veterinarians in mitigating risks.
Factors That Affect Veterinary Antibiotic Ranking and Usage

Table of Contents 1. Introduction: Why Antibiotic Ranking Matters Defining Antibiotic Rank Antibiotic ranking, exemplified by frameworks such as the Desirability of Outcome Ranking for the Management of Antimicrobial Therapy (DOOR MAT), is a comparative evaluative system used to classify antibiotics based on clinical, microbiological, and stewardship-related outcomes. These ranking models integrate multiple criteria to provide a balanced assessment of antibiotic desirability and therapeutic appropriateness. Drawing from these frameworks, antibiotic ranking generally incorporates four key dimensions: Clinical Effectiveness or Activity:The ranking prioritizes treatments containing an active agent against the infecting pathogen. The DOOR MAT framework, for instance, evaluates whether a therapeutic choice is active or inactive against a confirmed organism, directly linking activity with patient outcomes. Resistance Rates and Spectrum of Activity:Ranking methods also consider the relationship between antimicrobial spectrum and resistance potential. Narrow-spectrum antibiotics, such as penicillin V, amoxicillin, and dicloxacillin, are typically ranked higher in desirability because they exert less selective pressure on the microbiota. In contrast, broad-spectrum agents, including third-generation cephalosporins (e.g., ceftriaxone), fluoroquinolones (e.g., ciprofloxacin, levofloxacin), and carbapenems (e.g., meropenem), tend to rank lower due to their greater ecological impact and higher risk of promoting resistance. Frameworks like the Antibiotic Spectrum Index (ASI) quantify these differences by assigning spectrum scores to each agent, enabling objective comparison and stewardship-based decision-making. Safety Profile and Cost Considerations:Although not always included in the primary ranking score, additional factors—such as toxicity, cost, availability, ease of administration, and drug–drug interactions—inform antibiotic appropriateness. For example, antibiotics within the WHO “Access” category are generally considered safer and more affordable options. Stewardship Importance:Ranking frameworks also evaluate antibiotics based on their role in antimicrobial stewardship, emphasizing the use of agents that balance clinical efficacy with the minimization of resistance development. The Role of Antibiotic Ranking in Clinical and Policy Decisions Antibiotic ranking systems are essential tools in clinical governance and global health policy, particularly in addressing the growing threat of antimicrobial resistance (AMR). Guiding Treatment Guidelines and Formulary Decisions:Ranking methods provide a structured and quantitative means of assessing antibiotic appropriateness. They assist institutional committees in developing treatment protocols by integrating local surveillance data, such as antibiograms, to match antibiotic selection with resistance profiles. The guiding principle is to ensure that antibiotic therapy—when justified—is both targeted and evidence-based. Narrow-spectrum agents are therefore prioritized over broad-spectrum alternatives whenever clinical efficacy is maintained. Informing AMR Policy and Stewardship Programs:Because 30% to 50% of antibiotic prescriptions are considered inappropriate, ranking frameworks are vital for identifying and reducing misuse. They help stewardship teams evaluate prescribing patterns and adjust therapeutic choices to minimize selective pressure on bacterial populations. Global policy initiatives, such as the WHO’s Access, Watch, Reserve (AWaRe) classification, further support the rational distribution of antibiotic use. Similarly, the WHO Bacterial Priority Pathogens List (BPPL) ranks pathogens based on their resistance threat, thereby guiding research and development (R&D) priorities for new antimicrobials. The WHO AWaRe Classification: A Model of Stewardship-Based Ranking The WHO AWaRe Classification, launched in 2017, is a globally recognized model designed to improve antibiotic use and curb resistance by categorizing antibiotics according to their public health importance and resistance risk. The system divides antibiotics into three stewardship-based categories: Access:These antibiotics pose a lower risk of resistance and are recommended as first- or second-line treatments for common infections. They are typically safe, affordable, and suitable for wide availability without restriction. Watch:Broad-spectrum agents associated with higher resistance potential fall into this group. Their use should be limited to specific indications or cases where Access antibiotics are ineffective. Reserve:These represent last-resort antibiotics reserved for infections caused by multidrug-resistant organisms. Their use should be tightly controlled and guided by expert consultation. The WHO recommends that more than 60% of national antibiotic consumption should derive from the Access group. The WHO recommends that more than 60 percent of national antibiotic consumption should come from the Access group. Organizations such as the Global Antibiotic Research and Development Partnership (GARDP) have proposed refinements to the AWaRe framework, particularly regarding Reserve antibiotics, to keep the system clinically relevant and responsive to evolving global resistance patterns. 2. Clinical Efficacy and Spectrum of Activity Broad vs. Narrow Spectrum: How Therapeutic Coverage Shapes Rank Antibiotic ranking frameworks prioritize therapeutic coverage based on the dual goals of effective patient treatment and antimicrobial stewardship. The general preference is for narrow-spectrum agents over broad-spectrum agents, provided that patient outcomes are not compromised. Key aspects of how spectrum shapes rank include: Minimizing Selective Pressure:Effective stewardship requires evaluating not only the efficacy of an antibiotic but also its spectrum of activity to minimize selective pressure, which drives the emergence of future resistance. Defining Desirability:Frameworks such as the Desirability of Outcome Ranking for the Management of Antimicrobial Therapy (DOOR MAT) classify treatment selection according to two primary principles: Treatments containing an active agent are more desirable than inactive ones; and Narrow-spectrum antibiotics are more desirable than broad-spectrum agents when equally effective. Ranking Overtreatment:In systems such as DOOR MAT, inappropriate therapy is defined as treatment that is active but unnecessarily broad in spectrum, known as overtreatment. Overtreatment is stratified into ordered categories, such as slight overtreatment, moderate overtreatment, and severe overtreatment. All of these rank lower than ideal treatment, which is the narrowest active therapy. Quantitative Measurement:The Antibiotic Spectrum Index (ASI) provides a quantitative measure of antibiotic exposure based on spectrum of activity. Each antibiotic receives a score from 1 to 13, allowing classification into four categories: Narrow (1–2), Intermediate (3–4), Broad (5–7), and Very Broad (≥8).Studies using ASI have shown that the mean ASI increases with the level of care, rising from Narrow in outpatients to Broad and Very Broad in hospitalized or ICU patients, illustrating the link between clinical setting and antibiotic breadth. Mechanism of Action: Bactericidal vs. Bacteriostatic Distinction The mechanism of action determines an antibiotic’s pharmacological effect on its target pathogen and is a major factor influencing its ranking and clinical appropriateness. General Mechanism:Antibiotics are selectively cytotoxic to microbial cells. They typically act either by disrupting the peptidoglycan cell wall or by interfering with essential metabolic processes within the bacterial
Bioguard in the Wuxi International Conference Center

On November 1st, Bioguard Corporation Participated in the 4th Asian Small Animal Specialist Conference and the 2nd China International Exotic Pet Conference., which successfully concluded after three days of events at the Wuxi International Conference Center, the conference was a significant platform for learning and exchange among international veterinarians. It drew numerous domestic and international experts, scholars, and industry leaders in pet medicine. A total of 6,229 veterinarians participated, making it a significant event. Bioguard features several prominent speakers, including: – Dr. Luo Shengteng, the Director of Taipei Hongcheng Animal Hospital, is known as the “Cat Doctor.” – Dr. Lin Zhengyi, who participated via live interaction and distributed copies of his published works. – Dr. Dong Guangzhong, a veterinary parasitologist from National Chung Hsing University in Taiwan, hosted a signing event for his parasitology atlas. -Dr. Dieter Everaet, a specialist in exotic animals, gave an amazing lecture titled “Dental Disease in Rabbits and Rodents.”. Each expert shares unique insights into exotic pet care, enhancing the excitement and elevating the event’s atmosphere.
What Does “Antibiotic Rank” Mean in Veterinary Medicine?

Table of Contents 1. Why “Antibiotic Rank” Matters in Veterinary Medicine Defining antibiotic ranking in veterinary antimicrobial stewardship In veterinary antimicrobial stewardship, antibiotic ranking refers to the structured classification of antimicrobial agents based on their public health importance, therapeutic value in animals, and the potential risk they pose for driving antimicrobial resistance. These ranking systems create a shared scientific framework that identifies which antibiotics should be preserved, which require caution, and which remain suitable as first-line options for veterinary use. International authorities guide this process. The World Organisation for Animal Health (WOAH, formerly OIE) publishes a List of Antimicrobial Agents of Veterinary Importance, while the European Medicines Agency’s Antimicrobial Advice Ad Hoc Expert Group (AMEG) categorizes antibiotics into risk-based groups intended to harmonize stewardship across the European Union. Collectively, these systems promote the rational and judicious use of antimicrobials in animals. How ranking frameworks help veterinarians choose antibiotics responsibly Antibiotic ranking systems function as practical decision tools for veterinarians. By stratifying antimicrobials according to their criticality, these frameworks: Highlight drugs that require strict restraint Direct clinicians toward lower-risk first-line options Support evidence-based prescribing For example, the AMEG classification guides veterinarians by designating high-risk agents in Category B (Restrict), which should only be used when no suitable alternatives exist. This structured hierarchy helps balance animal welfare with the responsibility to limit the emergence of antimicrobial resistance. One Health context: linking animal use to human and environmental resistance risks Antibiotic ranking is fundamentally shaped by the One Health perspective, which recognizes that the health of humans, animals, and the environment forms an interconnected system. Antimicrobial use in animals can influence resistance patterns in human pathogens through several pathways, including food production, environmental contamination, and direct human–animal interactions. Because antibiotic use in one sector can amplify resistance risks across the entire ecosystem, global organizations emphasize the need for coordinated stewardship across species. Updated scientific opinions, such as those issued by the EMA’s AMEG or WOAH’s expert panels, explicitly address how antibiotic choices in veterinary medicine affect both animal health and public health. In this context, responsible prescribing in animals becomes an essential contribution to preserving the long-term effectiveness of antimicrobial agents worldwide. (European Medicines Agency & AMEG, 2024; World Organisation for Animal Health, 2021). 2. What “Antibiotic Rank” Means in Veterinary Medicine In veterinary medicine, antibiotic ranking refers to the structured classification of antimicrobial agents based on their therapeutic importance, public health relevance, and the potential risks associated with their use. These systems create a risk-based categorization framework that guides veterinarians in the rational use of antimicrobials across all animal sectors. International bodies have established standardized frameworks to support responsible prescribing. The WOAH (formerly OIE) List of Antimicrobial Agents of Veterinary Importance and the AMEG classification are two of the most widely recognized systems. Both frameworks rely on specific scientific criteria to determine how antibiotics should be prioritized for use in animals. Key Criteria Used in Veterinary Antibiotic Ranking Public health importance of the antibiotic classRanking systems evaluate how the use of a particular antimicrobial in animals may affect human health. Antibiotic classes that are critically important to human medicine are placed into higher-risk categories, emphasizing that their veterinary use should be limited and carefully justified. Risk of resistance transfer to humansAntimicrobial resistance moves across species, ecosystems, and food systems. Ranking frameworks incorporate the likelihood that resistance genes selected in animals could reach human populations through direct contact, the food chain, or environmental pathways. AMEG specifically provides updated scientific advice that incorporates risks to both public health and animal health. Therapeutic necessity in animalsSome antimicrobial classes are essential for maintaining animal welfare. The WOAH list identifies drugs of clear veterinary importance, recognizing that certain infections in livestock and companion animals cannot be effectively managed without them. Ranking systems balance this therapeutic need with the potential broader risks associated with drug use. • Availability of suitable alternativesAntibiotic ranking promotes responsible antimicrobial selection by encouraging the use of lower-risk alternatives whenever they provide adequate therapeutic benefit. By defining clear categories, frameworks help veterinarians reserve higher-risk or human-critical agents for cases where no appropriate alternatives exist, supporting the rational use of antimicrobials in daily practice. 3. Understanding the EMA AMEG Antibiotic Categorization System The Antimicrobial Advice Ad Hoc Expert Group (AMEG) of the European Medicines Agency (EMA) defines a risk-based antibiotic categorization system that guides the prudent and responsible use of antimicrobials in animals. This system evaluates both the public health consequences of antimicrobial resistance (AMR) arising from veterinary use and the therapeutic necessity of these drugs for maintaining animal health. AMEG stratifies antimicrobials into four categories, ranging from A to D, with Category A representing the highest public health risk and Category D representing the lowest. Category Risk Level / AMEG Meaning Veterinary Directive Examples of Antibiotic Classes Use Context Category A – Avoid Highest risk, reserved exclusively for human medicine Not authorized for veterinary use in the EU. May only be used in companion animals in exceptional cases and only under the prescribing cascade. Absolutely prohibited in food-producing animals. Carbapenems (meropenem, doripenem), glycopeptides (vancomycin), oxazolidinones (linezolid), select advanced cephalosporins/penems reserved for human therapy. Human-only antibiotics where veterinary use could severely compromise public health. Category B – Restrict Critically important for human medicine Use only when no effective Category C or D alternatives exist. Veterinary use must be justified. Diagnostic requirement: Antimicrobial susceptibility testing (AST) recommended before use. Fluoroquinolones (enrofloxacin, marbofloxacin), 3rd–4th gen cephalosporins (ceftiofur, cefquinome), polymyxins (colistin, polymyxin B). Category C – Caution Medium risk; human alternatives available but limited veterinary alternatives Use only when Category D drugs would not provide adequate clinical efficacy. Macrolides (tylosin, tulathromycin), aminoglycosides except spectinomycin (gentamicin, streptomycin), 1st–2nd gen cephalosporins, aminopenicillins with β-lactamase inhibitors (amoxicillin–clavulanic acid). Provides options for cases where first-line agents may be ineffective. Category D – Prudence Lowest public-health risk Recommended as first-line options when clinically appropriate. Avoid overuse, long treatment durations, and routine group treatments unless individual treatment is not feasible. Penicillins (amoxicillin, benzylpenicillin), tetracyclines (oxytetracycline, doxycycline), sulfonamides including potentiated combinations (sulfadiazine + trimethoprim).
Bioguard Office Relocation and Inauguration Ceremony

Bioguard relocated to its new office at the beginning of September and held a brand-new identity and office inauguration ceremony this October 4th, inviting distinguished guests from various fields to join the celebration. At the event, in addition to unveiling the new logo, guests also had the opportunity to tour the new office and the state-of-the-art Animal Disease Testing Center laboratory, the Bioguard Testing Center laboratory meets ISO17025 standards, ensuring the accuracy and impartiality of test results, providing veterinarians with reliable diagnostic support. We would like to express our gratitude to all the guests who attended today’s event and your presence has made Bioguard shine even brighter and in the future, Bioguard will continue to provide superior services and protect every animal through the power of biotechnology.
Understanding Antibiotic Classifications: A Comprehensive Guide
Antibiotics are essential tools in modern medicine, used to combat bacterial infections that could otherwise lead to severe health issues or even death. To use these powerful drugs effectively, it’s crucial to understand the different classifications of antibiotics, which are based on their chemical structure, mechanism of action, and spectrum of activity. This article explores the main classifications of antibiotics, providing an overview of their uses and how they work. Beta-Lactam Antibiotics Examples: Penicillins, Cephalosporins, Carbapenems, Monobactams Mechanism of Action: Beta-lactam antibiotics work by inhibiting the synthesis of bacterial cell walls. They target the penicillin-binding proteins (PBPs) that are crucial for forming peptidoglycan, a key component of the bacterial cell wall. By disrupting this process, beta-lactams weaken the bacterial cell wall, leading to cell lysis and death. Uses: These antibiotics are widely used to treat a variety of infections, including respiratory tract infections, urinary tract infections, skin infections, and more. Penicillins are often the first line of defense against many common bacterial infections. Macrolides Examples: Erythromycin, Azithromycin, Clarithromycin Mechanism of Action: Macrolides inhibit bacterial protein synthesis by binding to the 50S ribosomal subunit, preventing the translocation of peptides. This action effectively stops the bacteria from growing and multiplying. Uses: Macrolides are particularly useful for treating respiratory infections, such as pneumonia and bronchitis, as well as skin infections. They are also an alternative for patients allergic to penicillin. Tetracyclines Examples: Tetracycline, Doxycycline, Minocycline Mechanism of Action: Tetracyclines inhibit bacterial protein synthesis by binding to the 30S ribosomal subunit. This prevents the attachment of aminoacyl-tRNA to the mRNA-ribosome complex, thereby halting protein synthesis and bacterial growth. Uses: Tetracyclines are used to treat a variety of infections, including skin infections, respiratory tract infections, and urinary tract infections. Aminoglycosides Examples: Gentamicin, Amikacin, Tobramycin Mechanism of Action: Aminoglycosides bind to the 30S subunit of bacterial ribosomes, leading to the misreading of mRNA. This causes the bacteria to produce faulty proteins, ultimately leading to cell death. Uses: These antibiotics are often used to treat serious infections caused by Gram-negative bacteria, such as sepsis, endocarditis, and complicated urinary tract infections. Due to their potential for toxicity, they are usually reserved for severe infections. Fluoroquinolones Examples: Ciprofloxacin, Levofloxacin. Mechanism of Action: Fluoroquinolones inhibit bacterial DNA gyrase and topoisomerase IV, enzymes critical for DNA replication and transcription. By disrupting these processes, fluoroquinolones prevent bacterial cell division and lead to cell death. Uses: Fluoroquinolones are used to treat a variety of infections, including respiratory tract infections, urinary tract infections, gastrointestinal infections, and skin infections. Sulfonamides Examples: Sulfamethoxazole, Sulfadiazine Mechanism of Action: Sulfonamides inhibit dihydropteroate synthase, an enzyme involved in folate synthesis in bacteria. Folate is necessary for DNA synthesis and cell division, so its inhibition leads to bacterial growth arrest. Uses: Sulfonamides are commonly used in combination with trimethoprim (e.g., as co-trimoxazole) to treat urinary tract infections, respiratory infections, and some types of diarrheas. Glycopeptides Examples: Vancomycin Mechanism of Action: Glycopeptides inhibit bacterial cell wall synthesis by binding to the D-alanyl-D-alanine termini of cell wall precursor units. This prevents the cross-linking of peptidoglycan chains, which is essential for bacterial cell wall strength and rigidity. Uses: Glycopeptides are used primarily to treat serious Gram-positive infections, especially those caused by methicillin-resistant Staphylococcus aureus (MRSA) and other resistant organisms. Oxazolidinones Examples: Linezolid, Tedizolid Mechanism of Action: Oxazolidinones inhibit protein synthesis by binding to the 50S subunit of the bacterial ribosome, preventing the formation of a functional initiation complex for protein translation. Uses: Oxazolidinones are used to treat serious infections caused by Gram-positive bacteria, including MRSA and vancomycin-resistant enterococci (VRE).
September Free webinar : Fluid and Electrolyte Balance.

Join us for a Continuous Learning with Bioguard session: Fluid and Electrolyte Balance 🔵Date: September 26, 2024 🔵Time: 8PM – 9PM (GMT+8) Present in ENGLISH Please click on the link below for registration: https://docs.google.com/forms/d/e/1FAIpQLScHxeNGC00vzXBL2p5Ekd-zr5EwotWPL_3z-QHRBiVNQvrfKA/viewform 🔵ABOUT THE WEBINAR: Electrolyte balance is crucial in veterinary clinical pathology as it influences cellular function, fluid homeostasis, and overall health. Electrolytes such as sodium, potassium, calcium, and chloride are essential in maintaining osmotic balance, acid-base equilibrium, and neuromuscular excitability. Disruptions in electrolyte levels can manifest as various clinical conditions, including dehydration, renal dysfunction, and metabolic imbalances. Accurate assessment through serum or plasma electrolyte measurements helps diagnose underlying disorders and guide therapeutic interventions. Veterinary practitioners must adeptly interpret these findings to optimize patient care, ensuring the restoration and maintenance of electrolyte equilibrium for effective management of critical and chronic conditions. 🔴ABOUT THE SPEAKER: Dr. Lin got her D.V.M. degree from National Taiwan University and his Ph.D. from the College of Biological Science and Technology, National Chiao-Tung University. She is a professor in the Department of Veterinary Medicine and director of Zoonosis Research Center, National Taiwan University. In addition, she is a former director of the Animal Disease Diagnostic Center, National Taiwan University. Her specialties include Veterinary Clinical Microbiology, Immunology, and Animal Cancer Biology and Therapeutic Development.
Experience the difference of expert care and peace of mind using the Bioguard rapid test kit.

At Makati Dog and Cat Hospital and at Fil-Chinese Animal Clinic your pet’s health is their priority. In addition to initial tests on the patient’s condition, they also employ the #bioguard rapid test kit, enabling them to provide prompt and effective care. #bioguard is a Taiwan manufacturer of rapid test kits for companion and exotic animals and Some of the test kits available in the market are #cpv Ag, #CDV Ag, #fpv Ag, #FCoV Ag, and #FeLV Ag/#FIV Ab to name a few. Experience the difference of expert care and peace of mind using the #bioguard rapid test kit. Contact us or directly at: service@bioguard.com.tw , to learn more about #bioguard and the list of available #rapidtestkit in the Philippine market.
WE ARE MOVING

Exciting Announcement!! Starting Sep 1, 2024, Bioguard Corporation will be relocating to a new office space that boasts state-of-the-art facilities and a more comfortable work environment. The new space will better cater to our needs and ensure a great working environment for all of us. Please be assured that this address change will not affect any ongoing work. We’re truly grateful for your ongoing support and determined to serve you with greater efficiency Our new address will be: 6F, No.25, Wugong 5th Rd., Xinzhuang Dist., New Taipei City 248020, Taiwan
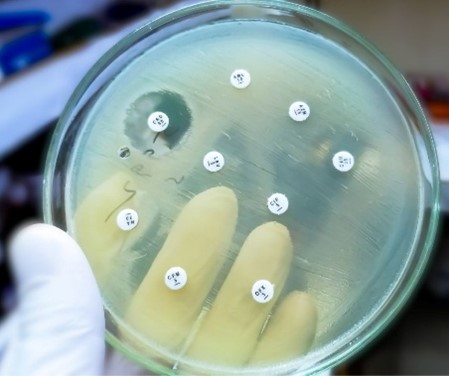
Get Ready for Bioguard new product Reveal at London Vet Show 2024!

Exciting Announcement!! Bioguard is exciting to announce our participation in the #LondonVetShow2024! Join us for an exclusive preview of our new product “new Antibiotic Susceptibility Test Analyzer”, designed to enhance animal healthcare. But First , Do you know what Antibiotic Susceptibility Testing is? Antibiotic Susceptibility Testing (AST) determines which antibiotics are most effective against bacterial infections in animals. It’s vital for choosing the right treatment. Please join Bioguard at the London Vet Show for a sneak peek of this New product and see how the process work. We can’t wait to share our latest advancements and connect with veterinary professionals. Date: November 14-15, 2024 Location: London Vet Show Stay tuned for more updates, and see you there!
