Trinh Mai Nguyen Tang
Feline panleukopenia virus (FPV) is a single-stranded DNA virus that causes feline leukopenia disease. It belongs to the family Parvoviridae, and is also known as Parvo in cats [1]. In the early years of the twentieth century, the first cases of FPV were reported in cats [2]. This is a common and dangerous disease in cats worldwide that occurs seasonally, especially from early summer to autumn with the highest peak in July, August, and September [3]. The virus usually resides in the everyday items of cats such as cages, beds, food bowls, etc [4]. Moreover, it has high resistance to physical factors and disinfectants, they can still survive for 30 minutes at temperatures up to 560C, and can even survive in contaminated environments for months or even years, which is why FPV is highly lethal and contagious in cats [4-5].
Transmission and clinical signs
FPV is spread by the fecal-oral route when cats come into contact with food, water, objects, or secretions that contain the virus. The virus enters and infects cells through the feline transferrin receptor (fTfR) [6-7].
Previous reports also showed that the two main types of cells that FPV replicate are small intestinal crypt cells and lymphoid cells [8-9]. The replication of virus leads to impaired white blood cell function, and bleeding in the small intestine and stomach is the cause of hemorrhagic diarrhea in cats [10]. According to Yen et al.’s report in 2021, the percentage of small intestine congested is 100% in a total of 8 cats examined (Figure 1A-B), and 75% of intestinal mucosal ulcerations appear. Meanwhile, there are 6 cats with gastric congestion in 75% (Figure 1C) and 2 cats with pneumonia accounting for 25% (Figure 1-D). In addition, other lesions were also detected, such as enlarged lymph nodes in the mesentery, and infarcted spleen [10]. These lesions can cause symptoms such as anorexia, fever, vomiting, and hair loss in the early stages [11], followed by diarrhea, severe dehydration, electrolyte imbalance, hypoglycemia, hemorrhage or sepsis and endotoxins in the blood cause rapid death in cats if not treated promptly [12-15]. Furthermore, in some kittens, there is damage to the central nervous system such as hydrocephalus, and cerebellar hypoplasia [16].
According to Reif (1976), cats infected with FPV have an incubation period of 4 to 6 days [16], during infection can excrete virus-containing feces for up to 43 days, and they even can survive for more than 1 year in the lungs and kidneys [17]
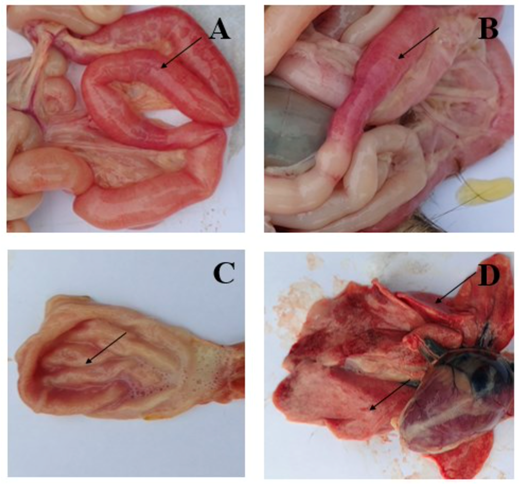
Figure 1. Symptoms of cat infection with FPV. A, B: Intestinal congestion, scattered or intermittent bleeding; C: Congested stomach contains a lot of fluid; D: Mild pneumonia [10]
FPV can cause infection in cats 3 to 5 months of age when infected with them or in unvaccinated cats, especially kittens, with an almost 90% chance of mortality due to maternally derived antibodies (MDAs) weakened [18]. MDAs may protect kittens against disease during the first weeks of life, and they are mainly transmitted through colostrum [19-20]. However, a kitten’s immunity will depend on factors such as the length of the lactation, the quality of the colostrum, and the amount of milk they are ingested [21].
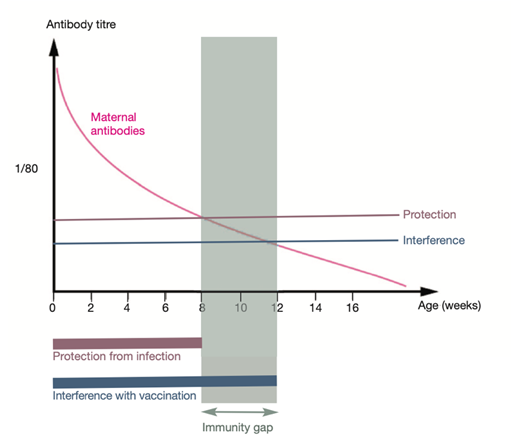
Figure 2. The immunity gap and maternally derived antibodies (MDAs) [24]
Although kittens all have antibodies inherited from the mother, these antibodies can only protect for 6-8 weeks, these antibodies gradually disappear and create an immunity gap that makes it easier for the virus to attack [19]. The immunological gap (Figure 2) is the distance that the maternally derived antibodies from the mother cat to the kittens are reduced. This gap usually occurs when kittens reach the age of 8-12 weeks and interferes with the development of immunization immunity [24]. There is evidence that there is seroconversion with long-term antibody titers after administration of modified live vaccines (MLV) in kittens lacking MDAs compared with kittens receiving MDAs [22–23]. When MDA titres ≥1:10 as measured by inhibition of coagulation (HI), MLV vaccination should not be given because of seroconversion [19, 24].
Diagnosis
FPV can be difficult to diagnose accurately because it has similar symptoms to feline immunodeficiency virus (FIV) infection. Symptoms of feline leukemia virus (FeLV) infection and pancreatitis vary depending on the extent of the infection.
FPV detection using PCR
There are many methods to detect the presence of FPV in the cat. One of the most widely used and informative methods today is PCR testing, which can use whole blood samples or feces [26].
FPV detection using ELISA method or Rapid test kit
The ELISA method can detect the presence of FPV-specific antigens in cat feces. When cats show clinical symptoms, they can be tested by ELISA or using the FPV antigen rapid test kit. The FPV Antigen test kit made by Bioguard Corporation is simple, fast, and accurate with a specificity and sensitivity of 92.54%.
FPV detection using blood test method
One of the methods of FPV diagnosing is to count white blood cells through a blood test. Other reports have also shown that markedly reduced leukocyte counts in hematologic parameters are very common in cats with FPV usually below 3000 and may reach less than 200/mm3 [3].
Prevention and Treatment
Because the FPV virus can survive for a long time in the environment and has high resistance to physical factors and disinfectants so it is necessary to clean the barn, shelter, and food bowl after the cat has been infected. In addition, it is necessary to isolate infected cats to avoid infecting other cats and becoming an outbreak
FPV in cats is a dangerous disease with no cure. All treatment for leukopenia in cats is for symptomatic treatment. Although there is no cure for feline leukopenia, if detected in time, the symptoms can be treated and the cat can recover.
Prevention
The way to prevent cats from contracting FPV is vaccination. Atteunuated live virus vaccines can be used, but should not be given to kittens < 4 weeks of age, immunocompromised, or pregnant cats.
References
- Parrish, C. R. (1990). Emergence, natural history, and variation of canine, mink, and feline parvoviruses. Advances in virus research, 38, 403-450.
- Verge, J., & Christoforoni, N. (1928). La gastroenterite infectieuse des chats; est-elle due à un virus filtrable. CR Seances Soc Biol Fil, 99, 312.
- REIF, J. S. (1976). Seasonally, natality and herd immunity in feline panleukopenia. American Journal of Epidemiology, 103(1), 81-87.
- Rehme, T., Hartmann, K., Truyen, U., Zablotski, Y., & Bergmann, M. (2022). Feline Panleukopenia Outbreaks and Risk Factors in Cats in Animal Shelters. Viruses, 14(6), 1248.
- Parker, J. S., Murphy, W. J., Wang, D., O’Brien, S. J., & Parrish, C. R. (2001). Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells.Journal of Virology, 75(8), 3896-3902.
- Parker, J. S., Murphy, W. J., Wang, D., O’Brien, S. J., & Parrish, C. R. (2001). Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells.Journal of Virology, 75(8), 3896-3902.
- Hueffer, K., Govindasamy, L., Agbandje-McKenna, M., & Parrish, C. R. (2003). Combinations of two capsid regions controlling canine host range determine canine transferrin receptor binding by canine and feline parvoviruses. Journal of Virology, 77(18), 10099-10105.
- Anderson, G. J., Powell, L. W., & Halliday, J. W. (1990). Transferrin receptor distribution and regulation in the rat small intestine: effect of iron stores and erythropoiesis.Gastroenterology, 98(3), 576-585.
- Chan, L. N., & Gerhardt, E. M. (1992). Transferrin receptor gene is hyperexpressed and transcriptionally regulated in differentiating erythroid cells.Journal of Biological Chemistry, 267(12), 8254-8259.
- Yến, N. T., Sơn, N. V., Ngọc, N. T., Lê Văn Hùng, N. T. G., & Hưng, P. Q. NGHIÊN CỨU MỘT SỐ BIẾN ĐỔI BỆNH LÝ VÀ ĐẶC TÍNH SINH HỌC CỦA FELINE PANLEUKOPENIA VIRUS PHÂN LẬP TRÊN MÈO Ở HÀ NỘ
- Ichijo, S. (1976). Clinical and hematological findings and myelograms on feline panleukopenia.
- Greene CE, Addie D. Feline parvovirus infection. In: Greene CE, ed.Infectious Diseases of the Dog and Cat. 3rd St. Louis, MO: Elsevier; 2006:78–86.
- Rehme, T., Hartmann, K., Truyen, U., Zablotski, Y., & Bergmann, M. (2022). Feline Panleukopenia Outbreaks and Risk Factors in Cats in Animal Shelters. Viruses, 14(6), 1248.
- Sykes, J. E. (2014). Feline panleukopenia virus infection and other viral enteritides. Canine and Feline Infectious Diseases, 187.
- Sykes, J. E. (2014). Feline panleukopenia virus infection and other viral enteritides.Canine and Feline Infectious Diseases, 187.
- Url, A., Truyen, U., Rebel-Bauder, B., Weissenböck, H., & Schmidt, P. (2003). Evidence of parvovirus replication in cerebral neurons of cats. Journal of clinical microbiology, 41(8), 3801-3805.
- Bentinck-Smith, J. (1949). Feline panleukopenia (feline infectious enteritis)-a review of 574 cases. North Am Vet, 30, 379-384.
- Jakel, V., Cussler, K., Hanschmann, K. M., Truyen, U., König, M., Kamphuis, E., & Duchow, K. (2012). Vaccination against feline panleukopenia: implications from a field study in kittens. BMC Veterinary Research, 8(1), 1-8.
- Scott, F. W., Csiza, C. K., & Gillespie, J. H. (1970). Maternally derived immunity to feline panteukopenia. Journal of the American Veterinary Medical Association, 156, 439-453.
- Chappuis, G. (1998). Neonatal immunity and immunisation in early age: lessons from veterinary medicine. Vaccine, 16(14-15), 1468-1472.
- Cao, Q., Chen, Y. C., Chen, C. L., & Chiu, C. H. (2020). SARS-CoV-2 infection in children: Transmission dynamics and clinical characteristics. Journal of the Formosan Medical Association, 119(3), 670.
- Gueguen, S., Martin, V., Bonnet, L., Saunier, D., Mähl, P., & Aubert, A. (2000). Safety and efficacy of a recombinant FeLV vaccine combined with a live feline rhinotracheitis, calicivirus and panleucopenia vaccine. Veterinary Record, 146(13), 380-381.
- Gore, T. C., Lakshmanan, N., Williams, J. R., Jirjis, F. F., Chester, S. T., Duncan, K. L., … & Sterner, F. J. (2006). Three-year duration of immunity in cats following vaccination against feline rhinotracheitis virus, feline calicivirus, and feline panleukopenia virus. Veterinary Therapeutics, 7(3), 213.
- Gaskell, R. M. (1989). Vaccination of the young kitten. Journal of Small Animal Practice, 30(11), 618-624.
- Truyen, U., Addie, D., Belák, S., Boucraut-Baralon, C., Egberink, H., Frymus, T. & Horzinek, M. C. (2009). Feline panleukopenia. ABCD guidelines on prevention and management.Journal of Feline Medicine & Surgery,11(7), 538-546.
- Schunck, B., Kraft, W., & Truyen, U. (1995). A simple touch-down polymerase chain reaction for the detection of canine parvovirus and feline panleukopenia virus in feces.Journal of virological methods,55(3), 427-433.
Lappin, M. R., Andrews, J., Simpson, D., & Jensen, W. A. (2002). Use of serologic tests to predict resistance to feline herpesvirus 1, feline calicivirus, and feline parvovirus infection in cats. Journal of the American Veterinary Medical Association, 220(1), 38-42.

