(https://share.google/images/epa3Ydpxlht3J77m3)
The Invisible Pandemic
Antimicrobial resistance (AMR) is now widely recognized as one of the most formidable global health threats of the 21st century. It fundamentally undermines the efficacy of existing antibiotics and jeopardizes the very foundations of modern medicine — from routine surgical safety to cancer chemotherapy and neonatal care. The relentless rise of resistance has transformed once-treatable bacterial infections into persistent, sometimes untreatable, conditions, eroding decades of medical progress.
This growing crisis is fueled by the uncontrolled and often unjustified use of antimicrobial agents across human healthcare, veterinary practice, and agriculture. Without coordinated global action, AMR could evolve into the next true pandemic. The gravity of this issue is underscored by the World Economic Forum, which classifies antibiotic resistance as a transnational risk exceeding the capacity of any single organization or nation to manage alone.
The global outlook is dire. Projections indicate that annual mortality linked to resistant infections may exceed 10 million deaths by 2050, far surpassing the current estimated 700,000 deaths each year, underscoring the urgent need to decode the biological mechanisms that drive this phenomenon, including how resistance arises, adapts, and moves across species and environments (O’Neill, 2016).
At its core, AMR is an evolutionary challenge. The emergence and spread of resistance stem from genetic variation within bacterial populations and the selective pressures exerted by antibiotic exposure. Resistance traits can originate de novo, through spontaneous mutations or structural rearrangements during DNA replication, and subsequently proliferate via horizontal gene transfer (HGT) — the lateral exchange of genetic material between unrelated bacterial cells. HGT serves as a conduit for resistance genes to traverse species boundaries, enabling pathogens to acquire multi-drug resistance in a single event. This mechanism is particularly dominant among Gram-negative bacteria, where mobile genetic elements facilitate rapid adaptation.
Ultimately, the dissemination of resistance forms a complex ecological network linking human health, animal husbandry, and environmental reservoirs such as water, food, and sewage systems. Understanding these interconnections is essential to curbing the invisible pandemic of antimicrobial resistance before it surpasses our collective capacity to respond.
1.The Genetic Arms Race — How Bacteria Learn to Resist
Initiation of Resistance: Random Mutations and Natural Selection
At the evolutionary level, AMR emerges from the interplay between genetic diversity and selective pressure. Random mutations introduce variation, while antibiotics act as the environmental filter determining which variants survive.
Random Mutations: De Novo Innovation
Resistance can originate spontaneously through replication or repair errors in bacterial DNA. These heritable mutations are passed vertically to daughter cells. Although most mutations are harmful, a rare few confer survival benefits under antibiotic stress.
Bacteria’s rapid reproduction amplifies this effect: Escherichia coli can divide every 20 minutes, producing over 68 billion descendants in 12 hours. This massive population size raises the probability that resistant mutants arise purely by chance. The spontaneous mutation frequency for antibiotic resistance typically falls between 10⁻⁸ and 10⁻⁹ per generation—so in a population of 10⁹ cells, at least one resistant clone is likely to appear. Under oxidative or chemical stress, this rate may increase dramatically.
When antibiotics enter the environment, these mutations become the substrate of natural selection, allowing resistant lineages to thrive while susceptible ones vanish.
Natural Selection: The Filter of Survival
Antibiotics impose a strong selective pressure on microbial populations. Resistant bacteria, whether by mutation or gene acquisition, enjoy a survival advantage and replicate preferentially. Over successive generations, this produces a predominantly resistant community.
Resistance often entails a fitness cost, such as slower growth, yet under continuous exposure, the advantage of drug survival outweighs that penalty. Secondary “compensatory” mutations can subsequently restore growth efficiency, cementing resistant strains as dominant even in the absence of antibiotics.
Examples of Resistance Initiation
- Mycobacterium tuberculosis — Multidrug-Resistant TB (MDR-TB)
Resistance in M. tuberculosis arises almost exclusively via chromosomal mutations. For instance, rifampicin resistance results from point mutations in the rpoB gene encoding the β-subunit of RNA polymerase, which reduces drug binding. Progressive accumulation of such mutations can transform a susceptible infection into a pan-resistant strain.
- Staphylococcus aureus — MRSA Evolution
Resistance to penicillin G emerged in S. aureus as early as 1943, within a year of its introduction. Later, the acquisition of the staphylococcal cassette chromosome mec (SCCmec)—a mobile element conferring methicillin resistance—produced methicillin-resistant S. aureus (MRSA). This element imposed minimal fitness costs, enabling pandemic clones such as ST8:USA300 to thrive. S. aureus can also acquire rifampicin resistance through independent rpoB mutations, exemplifying the synergy of mutation and horizontal gene transfer.
- Escherichia coli — Multidrug-Resistant Strains
MDR E. coli often combine chromosomal and plasmid-borne mechanisms. Mutations in gyrA, gyrB, and parC yield fluoroquinolone resistance, while plasmids carrying CTX-M β-lactamases confer cephalosporin resistance. The pandemic clone ST131 illustrates this convergence, integrating both pathways to achieve broad resistance. Environmental surveillance reveals such strains in wastewater systems, highlighting how human waste streams perpetuate environmental dissemination.
Selective Pressure from Antibiotic Overuse
The misuse of antibiotics across medicine, agriculture, and aquaculture accelerates resistance evolution by continuously applying selective pressure.
In Healthcare
Antibiotics are indispensable but frequently misused.
- Inappropriate prescribing and patient demand sustain excessive use.
- Resistant mutants can dominate within days of therapy initiation.
- Hospitals, intensive care units, and nursing facilities become reservoirs of entrenched resistance.
In Agriculture and Food Production
The agricultural sector remains a powerful amplifier of AMR.
- Antibiotics are administered to healthy livestock for prophylaxis and growth promotion, and used in crops and aquaculture.
- This continuous exposure fosters resistance transfer between animals, humans, and the environment.
- The colistin resistance gene mcr-1, first detected in China, arose from decades of agricultural colistin use and has since spread worldwide.
- Food products themselves act as vehicles for resistant bacteria, underscoring the importance of a One Health approach that unites human, animal, and environmental management.
Visual Aid — Mutation-Driven vs. Acquired Resistance
| Mechanism | Description | Example Pathogen | Clinical Impact |
| Mutation (Vertical Transmission) | Random chromosomal alterations that modify target structures (e.g., ribosomal subunits, enzymes). | Mycobacterium tuberculosis | Drives multidrug- and pan-resistant TB; also mediates fluoroquinolone resistance via gyrA/gyrB mutations. |
| Gene Acquisition (Horizontal Transfer) | Uptake of external DNA elements—plasmids, integrons, transposons—from unrelated bacteria. | E. coli, S. aureus | Enables rapid multidrug resistance; disseminates via MGEs such as SCCmec in MRSA and ESBL plasmids in Enterobacteriaceae. |
In summary, resistance emerges through a dynamic interplay of mutation, selection, and misuse. These forces perpetuate an evolutionary arms race between medicine and microbes—a contest in which adaptation increasingly favors the latter.
2.Horizontal Gene Transfer — The Highway of Resistance
The evolutionary triumph of antibiotic resistance lies not solely in mutation but in the bacterial capacity for horizontal gene transfer (HGT) — the exchange of genetic material between cells that are not parent and offspring. Through HGT, resistance genes can leap across species, genera, and ecosystems, allowing bacteria to acquire multiple resistance determinants in a single genetic transaction. Among Gram-negative bacteria, particularly within Enterobacteriaceae, HGT represents the dominant route for the propagation of antimicrobial resistance.
Mechanisms of Horizontal Gene Transfer
Three primary mechanisms facilitate HGT: conjugation, transformation, and transduction. Each operates through distinct biological routes but shares the same evolutionary outcome — the acceleration of resistance dissemination far beyond what mutation alone could achieve.
| Mechanism | Simplified Description | Molecular Details and Impact |
| Conjugation | Direct cell-to-cell contact via a specialized pilus that transfers DNA from a donor to a recipient cell. | The most prevalent form of HGT. Conjugative plasmids often carry multiple resistance genes (R-plasmids). Once received, the new cell gains both resistance and the ability to further transfer the plasmid, perpetuating the spread. Conjugation can occur at rates several orders of magnitude higher than mutation. |
| Transformation | Uptake and incorporation of free DNA fragments from the environment. | Enables bacteria to scavenge genetic material from lysed cells. Transformation predates the divergence of Gram-positive and Gram-negative species, reflecting an ancient evolutionary adaptation. This process allows environmental DNA carrying resistance genes to become part of a bacterium’s genome. |
| Transduction | Bacteriophage-mediated transfer of bacterial DNA. | When viruses that infect bacteria (phages) accidentally package bacterial DNA instead of their own, they can inject this “hybrid” genetic material into new hosts. Though limited by host range, transduction facilitates the transfer of resistance genes between closely related bacteria. |
Plasmids and Integrons as Resistance Vehicles
The majority of transferable resistance genes reside on mobile genetic elements (MGEs) such as plasmids, transposons, and integrons, which serve as the molecular vehicles for antibiotic resistance.
- Plasmids are extra-chromosomal, self-replicating DNA circles that frequently encode multi-drug resistance (MDR) determinants. They are exchanged between species via conjugation and can carry dozens of resistance genes simultaneously.
- Example: The blaNDM-1 gene, which confers carbapenem resistance, achieved rapid global dissemination through conjugative plasmids, especially within Klebsiella pneumoniae. Likewise, the mcr-1 gene for colistin resistance, first detected in China, was mobilized by the ISApl1 transposon on IncI2 and IncX4 plasmids, a combination that facilitated its worldwide spread within less than a decade.
- Integrons act as genetic “platforms” that capture, rearrange, and express resistance gene cassettes. The Class 1 integron, found in up to 70% of Gram-negative bacteria, typically carries multiple resistance determinants. The integrase gene (intI1) is now widely recognized as a biomarker for antibiotic pollution in aquatic and soil environments due to its strong correlation with antibiotic usage intensity.
Case Study: ESBL Gene Transfer among Enterobacteriaceae
Plasmid mediated Extended Spectrum Beta Lactamase (ESBL) genes, particularly blaCTX-M, illustrate how horizontal gene transfer drives the expansion of resistance across clinical and environmental settings.
- Clonal spread in high-risk lineages: ESBL plasmids frequently circulate within high-risk E. coli clones such as ST131, a lineage recognized for its multidrug resistance and global prevalence in both hospital and community infections (Pitout and DeVinney, 2017).
- Gut microbiome as a conjugation hotspot: The human gut microbiome serves as an ideal environment for plasmid exchange, enabling Enterobacteriaceae to efficiently share ESBL-encoding mobile genetic elements and other resistance determinants (Kessler et al., 2022).
- Environmental dissemination: Environmental monitoring has detected ESBL-producing E. coli and Klebsiella pneumoniae in wastewater, hospital effluents, and surface waters, findings consistent with the environmental mobility of plasmids and transposons that carry antimicrobial resistance genes (Partridge et al., 2018; Husna et al., 2023). Notably, quinolone-resistant E. coli ST131 has been recovered from urban water systems in Barcelona, underscoring the bidirectional flow of resistant pathogens between clinical and aquatic environments (Pitout and DeVinney, 2017).
- Persistence in aquatic reservoirs: The longevity of ESBL plasmids in riverine and wastewater ecosystems demonstrates how anthropogenic waste streams sustain global circulation of resistant bacteria and their associated genetic elements (Husna et al., 2023; Partridge et al., 2018).
3. Beyond Hospitals — The Environmental Reservoir
Antimicrobial resistance extends far beyond clinical settings, forming a complex ecological network that links humans, animals, and the environment. The environment acts as both a reservoir and a bridge, enabling resistance genes to flow through microbial communities via mobile genetic elements (MGEs) that transcend ecological and species boundaries. Addressing this connectivity requires a One Health approach that unites medical, veterinary, and environmental disciplines.
Spread of Resistance Genes Through the Environment
Wastewater and Sewage
Wastewater systems represent one of the most concentrated environmental reservoirs for antimicrobial-resistant bacteria (ARB) and antimicrobial resistance genes (ARGs).
- Hospital and urban wastewater often contain antibiotic residues, ARGs, and ARB in high concentrations, creating ideal conditions for HGT.
- Wastewater treatment plants (WWTPs) are now recognized as hotspots for AMR emergence. While conventional treatment can reduce bacterial loads, it has limited efficacy in eliminating free-floating resistance genes.
- Studies have identified oxytetracycline and other antibiotic residues in industrial effluents, along with persistent ARGs such as sul1, sul2, and tet genes throughout the treatment process.
- An estimated 10¹⁹ bacteria carrying Class 1 integrons enter the UK environment annually via sewage sludge disposal, providing staggering evidence of the environmental scale of resistance dissemination.
Soil, Livestock, and Agriculture
Agricultural ecosystems are a significant source of resistance gene amplification and redistribution.
- Antibiotics are widely used in livestock and aquaculture not only for treatment but also for prophylaxis and growth promotion.
- Repeated exposure exerts selection pressure that fosters the emergence of resistant bacteria in animal gut flora and surrounding soil microbiomes.
- Resistance genes, including mcr-1, have been traced directly to agricultural antibiotic use, particularly colistin in pig and poultry farming in China, a practice now banned but whose legacy persists in environmental reservoirs.
- Soils treated with manure have shown high concentrations of ARGs and ARB, confirming agricultural waste as a conduit for environmental resistance flow.
The One Health Paradigm
The One Health approach acknowledges the interdependence of human, animal, and environmental health in the evolution and transmission of AMR.
- The WHO Global Action Plan on AMR emphasizes coordinated surveillance and policy integration across healthcare, veterinary, and environmental sectors.
- International bodies such as FAO, OIE (World Organisation for Animal Health), and WHO collaborate to harmonize resistance monitoring, laboratory standards, and data exchange.
- Understanding how resistance circulates between humans, animals, food, and the environment is vital for developing targeted interventions that prevent the reintroduction of resistance into clinical settings.
Companion Animals as Intermediate Hosts
While less frequently studied, companion animals represent potential intermediate hosts in the resistance network. Shared living spaces and microbiomes facilitate the bidirectional exchange of resistant bacteria between humans and pets. The gut flora of these animals, enriched by environmental or dietary exposure, may act as reservoirs of resistance genes later transferred to human-associated strains.
4. Crossing the Species Barrier — From Barnyard to Bedside
Zoonotic Transfer
Zoonotic transmission occurs when resistant bacteria from animals infect humans through direct contact, food consumption, or shared environments. The food chain is a particularly effective vehicle for resistance spread, as bacteria originating from agricultural antibiotic use survive processing and enter human populations.
- Livestock handlers face elevated risks of colonization by resistant pathogens, including methicillin-resistant Staphylococcus aureus (MRSA).
- The WHO Priority Pathogens List identifies fluoroquinolone-resistant Salmonella and Campylobacter as major public health threats.
- While macrolide-resistant Campylobacter jejuni may exhibit reduced transmission fitness in animals, their persistence in poultry and food products remains a critical concern.
- Collectively, these patterns highlight the bidirectional nature of resistance movement: from barnyard microbiomes to human gut ecosystems and back again.
AMR Surveillance in Veterinary Medicine
Recognizing the animal-human interface in resistance spread, international agencies have established surveillance and control frameworks:
- The Global Action Plan on AMR calls for harmonized global coordination among human, veterinary, and agricultural sectors.
- The OIE (World Organisation for Animal Health) develops international standards for antimicrobial usage monitoring and laboratory susceptibility testing.
- FAO and WHO jointly maintain the Codex Alimentarius Code of Practice to minimize and contain AMR in food production.
- Member States are urged to implement national action plans aligned with these standards, ensuring consistent reporting, data sharing, and policy enforcement across borders.
Summary
The movement of antimicrobial resistance across species boundaries demonstrates that human health cannot be isolated from animal and environmental systems. Antibiotic use in livestock creates selective pressures that enrich resistant bacteria in farm environments, food products, and waste streams. These organisms subsequently enter human populations through direct contact, contaminated meat, or shared ecological reservoirs, enabling continuous exchange of resistance genes between agricultural microbiomes and the human gut.
Global surveillance frameworks from WOAH, FAO, and WHO emphasize that controlling AMR requires coordinated stewardship across all sectors. By harmonizing antimicrobial usage monitoring, standardizing susceptibility testing, and regulating food production practices, these international systems aim to reduce selective pressure at the source and prevent zoonotic transfer. Ultimately, the animal–human interface represents a critical chokepoint in the resistance cycle, and strengthening oversight in this domain is essential to preserving antibiotic effectiveness worldwide.
5. The Role of Globalization — Travel, Trade, and Transmissions
Antimicrobial resistance is no longer confined to local clinical settings or national borders. The modern world — defined by unprecedented levels of human migration, medical tourism, global trade, and industrial interconnection — has created an ideal environment for resistant bacteria and their genes to circulate freely. AMR has thus evolved into a globalized epidemic, with the capacity to move faster than the very systems designed to contain it.
5.1 Global Mobility: The Human Vector
International travel plays a critical role in the worldwide dissemination of resistant pathogens.
- Medical tourism has become a key driver of transnational AMR spread. Patients seeking treatment abroad may acquire resistant strains during hospitalization and carry them home, sometimes asymptomatically.
- A 2019 prospective cohort study reported that more than 40 percent of travelers returning from regions with high AMR prevalence, particularly South and Southeast Asia, acquired ESBL-producing Enterobacteriaceae during their trip (Schaumburg et al., 2019).
- Carbapenem-resistant Klebsiella pneumoniae (CRKP) and Acinetobacter baumannii are now routinely identified in hospitals across Europe and North America, often linked to importation from endemic regions.
- Air travel facilitates the 24-hour global circulation of microbial lineages. For example, E. coli ST131, a high-risk clone carrying CTX-M β-lactamases, has been detected on every inhabited continent, demonstrating how human mobility effectively merges regional resistance networks into a global one (Frost et al., 2019).
5.2 Global Trade: From Food Chains to Pharmaceutical Supply Lines
Trade globalization has extended AMR’s reach beyond healthcare, embedding resistance in food, livestock, and supply chain ecosystems.
Food and Agricultural Trade
- The export and import of animal products (meat, seafood, dairy) allow resistant bacteria from one agricultural system to infiltrate others.
- Studies across Europe and Asia have identified colistin-resistant E. coli in imported poultry products, including isolates carrying the mcr-1 and mcr-3 genes, even in regions where colistin use in livestock has been banned. Detection of both mcr-1 and mcr-3 in poultry food isolates has been documented in Vietnam (Yamaguchi et al., 2018).
- Packaged seafood from aquaculture-rich nations such as Vietnam, Indonesia, and China frequently harbors multidrug-resistant Vibrio spp. and Aeromonas hydrophila, linking aquatic trade to global resistance dissemination.
Pharmaceutical Manufacturing and Waste
- The pharmaceutical supply chain itself contributes to environmental AMR. Manufacturing effluents from antibiotic production facilities contain high concentrations of active compounds, exerting selective pressure on environmental microbiota.
- Rivers in Hyderabad, India — home to one of the world’s largest antibiotic production hubs — have shown ciprofloxacin concentrations exceeding clinical serum levels, alongside high abundance of resistance genes such as qnr, sul, and blaTEM (Lübbert, Christoph, et al. 2017).
- These findings highlight a circular feedback loop: antibiotic manufacturing fuels resistance that ultimately undermines the very drugs being produced.
5.3 Migration of Genes: The Genomic Epidemiology Perspective
The modern field of genomic epidemiology has revealed that resistance genes themselves travel globally through mobile genetic elements (MGEs), independent of their bacterial hosts.
- Whole-genome sequencing (WGS) of clinical and environmental isolates has demonstrated identical resistance plasmids appearing in K. pneumoniae from hospitals in the UK, wastewater in India, and poultry farms in China.
- The blaNDM-1 carbapenemase gene, first identified in 2008 in a Klebsiella isolate from a patient in New Delhi, was found just two years later in clinical samples across 40 countries spanning five continents.
- These rapid, near-synchronous detections could not be explained by bacterial spread alone — instead, the plasmids carrying blaNDM-1 were transferred through international networks of trade, travel, and environmental contamination.
- This illustrates how genetic globalization has outpaced human surveillance systems, transforming resistance into a planetary-scale genomic event.
5.4 Case Examples: Global Routes of Resistance
| Pathogen / Gene | Primary Route of Dissemination | Geographic Origin | Current Global Status |
| blaNDM-1 (Carbapenemase) | Medical tourism, wastewater, global plasmid spread | India (2008) | Detected in >100 countries within 10 years |
| mcr-1 (Colistin resistance) | Livestock use and agricultural trade | China (2015) | Now found in human, animal, and environmental samples worldwide |
| E. coli ST131 (ESBL-producing) | International travel and fecal carriage | North America / Europe | Dominant pandemic clone across continents |
| K. pneumoniae ST258 (KPC) | Hospital transmission networks | USA / Israel | Established in healthcare systems globally |
Source: Mathers AJ, Peirano G, Pitout JD. (2015). Clin Microbiol Rev, 28(3):565–591.
5.5 The Network Effect — AMR in a Connected World
The interconnected nature of today’s world transforms local resistance problems into global crises.
- Urbanization and population density amplify transmission rates.
- Global supply chains ensure that resistant microbes can traverse oceans through goods, food, or waste.
- Digital sequencing databases, while improving surveillance, also reveal the daunting complexity of tracking resistance in real time.
- As genomic databases expand (e.g., NCBI Pathogen Detection, GISAID-AMR), resistance tracking has become a global data challenge as much as a public health one.
5.6 Mitigation Through Global Cooperation
No single country can contain AMR alone. The globalized nature of resistance requires equally globalized policy and data coordination:
- WHO’s Global Antimicrobial Resistance Surveillance System (GLASS) integrates AMR data from over 100 countries, creating a unified monitoring framework.
- The Tripartite Alliance (FAO–OIE–WHO) drives cross-sectoral harmonization of AMR standards.
- The World Bank estimates that global cooperation on AMR mitigation could prevent up to USD 100 trillion in cumulative economic losses by 2050.
- Investment in waste management, antibiotic stewardship, and genomic surveillance infrastructure remains essential to slow resistance momentum in both developed and developing nations.
5.7 Summary: From Local Mutations to Global Mobility
Antibiotic resistance has evolved from a molecular adaptation to a globalized ecosystem phenomenon. Genes once confined to a single hospital ward now traverse continents via trade, travel, and transboundary pollution. The genomic evidence is unequivocal: AMR does not respect borders. The only viable response must mirror its reach — through international cooperation, unified surveillance, and cross-sectoral stewardship that align under the One Health paradigm.
6. Fighting Back — Surveillance, Stewardship, and Science
Antimicrobial resistance (AMR) represents one of the greatest evolutionary and clinical challenges of our era. Yet, it is not an inevitable catastrophe. The same scientific ingenuity that gave rise to antibiotics now offers the tools to defend them. By combining surveillance, stewardship, and innovation, the global community can begin to slow — and potentially reverse — the spread of resistance.
6.1 Global Surveillance: Seeing the Invisible
Effective surveillance forms the backbone of every AMR containment strategy. It enables early detection of resistant strains, tracks emerging genetic variants, and informs public health policy.
- Integrated Genomic Monitoring: Whole-genome sequencing (WGS) and metagenomics have revolutionized AMR detection, enabling the tracing of resistant lineages across hospitals, farms, and ecosystems. For example, WGS of E. coli and Klebsiella isolates from wastewater and companion animals has uncovered previously unknown plasmid transfer routes.
- Global Systems: The WHO’s Global Antimicrobial Resistance Surveillance System (GLASS) and regional databases (such as EARS-Net in Europe) are improving real-time data integration.
- One Health Data Sharing: Incorporating environmental and veterinary samples into AMR databases ensures a more comprehensive picture. Genomic surveillance must now treat hospitals, food systems, and wildlife as a single microbial continuum.
6.2 Stewardship: Reclaiming Rational Antibiotic Use
Antibiotic stewardship focuses on the responsible and evidence-based use of antimicrobials across human, veterinary, and agricultural sectors.
- Clinical Stewardship Programs: Hospitals and clinics worldwide are implementing antibiotic review protocols, reducing inappropriate prescriptions by up to 30%.
- Veterinary Best Practices: In animal medicine, the cessation of growth-promoter antibiotics and adoption of pathogen-specific treatment protocols have significantly reduced resistance emergence in livestock populations.
- Education and Awareness: Behavioral change among healthcare professionals and pet owners remains essential. Responsible prescribing, diagnostic testing before treatment, and strict adherence to dosing guidelines are vital pillars of stewardship.
Stewardship is not about restriction — it is about precision: ensuring that every antimicrobial is used only when necessary, in the correct dosage, and for the appropriate duration.
6.3 Scientific Innovation: The Next Frontier
If the last century’s war on infection was chemical, the next will be biological and data-driven. New technologies are transforming how we detect, understand, and treat resistant pathogens.
AI and Predictive Genomics
Machine learning algorithms are now capable of predicting resistance phenotypes from genomic sequences, enabling pre-emptive diagnostics. AI-driven drug discovery platforms can screen millions of compounds in silico, accelerating the identification of new antibiotic scaffolds.
Bacteriophage and Microbiome Therapies
Phage therapy — the use of viruses that selectively infect bacteria — has re-emerged as a promising strategy against multidrug-resistant infections. Likewise, microbiome restoration and competitive exclusion strategies are being studied to outcompete resistant strains in the gut.
PET Imaging in Infection Tracking
Positron Emission Tomography (PET) is providing non-invasive visualization of infection sites and tracking of antimicrobial activity in vivo. Radiolabeled tracers, such as 18F-fluorodeoxyglucose (FDG), allow clinicians to distinguish between sterile inflammation and active bacterial infection. In veterinary medicine, PET imaging bridges the gap between experimental research and clinical diagnostics, supporting translational approaches under the One Health model.
Novel Therapeutics and Vaccines
Advances in genomics are yielding structure-guided drug designs, peptide-based antimicrobials, and vaccines targeting resistant pathogens. Combined with rapid diagnostic tools, these innovations mark a shift toward precision infection management.
6.4 Building Global Biosecurity: The Bioguard Perspective
At Bioguard Corporation, we believe that tackling AMR requires collaboration across disciplines — from clinical microbiology and veterinary diagnostics to genomic surveillance and public education.
Founded with the mission to safeguard animal and human health through innovation, Bioguard operates as a trusted partner to veterinary clinics, diagnostic laboratories, and research institutions worldwide. Our focus areas include:
- Advanced Diagnostic Solutions: Rapid molecular tests and culture-based systems for pathogen identification and antimicrobial susceptibility profiling.
- Veterinary Laboratory Network: Support for early detection of resistant zoonotic pathogens in companion animals and livestock, integrating veterinary diagnostics into the One Health AMR surveillance framework.
- Biosecurity Consulting: Tailored programs that help veterinary hospitals and animal facilities strengthen hygiene protocols, monitor environmental microbial loads, and reduce unnecessary antibiotic use.
- Research Collaboration: Partnerships with universities and international organizations to expand AMR genomic mapping and PET-based infection imaging in veterinary contexts.
By combining diagnostic precision, field expertise, and scientific collaboration, Bioguard contributes directly to global AMR containment efforts — bridging laboratory insight with practical action.
6.5 The Path Forward: From Awareness to Accountability
AMR is a shared problem that demands shared responsibility. Containing it requires coordinated action on three fronts:
- Detect: Strengthen surveillance networks and data transparency across human and veterinary sectors.
- Prevent: Implement stewardship protocols and infection control measures across all healthcare and agricultural systems.
- Innovate: Invest in new diagnostics, therapeutics, and real-time monitoring technologies.
Global resistance cannot be reversed by science alone — it requires public trust, sustained policy enforcement, and international solidarity. Every sample tested, every prescription reconsidered, and every diagnostic innovation contributes to slowing the invisible pandemic.
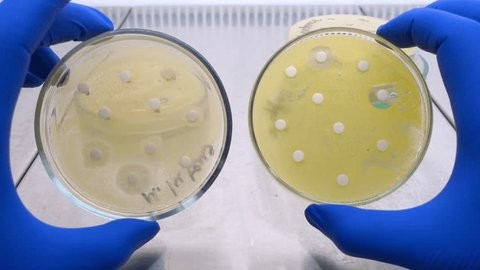
While responsible practices are critical, veterinarians can further strengthen their impact by leveraging advanced diagnostic tools. Rapid and accurate bacterial susceptibility testing supports evidence-based antibiotic selection, optimizing treatment outcomes and combating resistance spread.
To support responsible antibiotic use, Bioguard offers the MiniAST Veterinary Antibiotic Susceptibility Test Analyzer, a tool designed to help combat antimicrobial resistance with game-changing features:
| Feature | Benefit |
| Fast Results | Get results in just 6 hours, enabling swift and confident treatment. |
| Automated Interpretations | Instantly deliver precise susceptibility profiles, supporting faster, more informed clinical decisions and optimizing patient care. |
| Dual-Sample Testing | Double the efficiency with simultaneous analysis of two samples at once. |
| High Accuracy | Achieve an impressive 92% accuracy rate compared to traditional disc diffusion tests. |
https://www.bioguardlabs.com/miniast-product-en/
📌 Note for Veterinarians:
The MiniAST Veterinary Antibiotic Susceptibility Test Analyzer is available exclusively to licensed veterinarians and veterinary hospitals.
📩 How to Order MiniAST
To purchase MiniAST or request a quotation, please contact our sales team or email our customer service:
📧 service@bioguardlabs.com
☎️ Please include your hospital name and contact number so our sales representative can follow up with you directly.
Source:
World Organisation for Animal Health (WOAH) – Antimicrobial Resistance
