Generalized Alopecia with Vasculitis-Like Changes in a Dog with Babesiosis- Abstract Tasaki Y, Miura N, Iyori K, Nishifuji K, Endo Y, Momoi Y. J
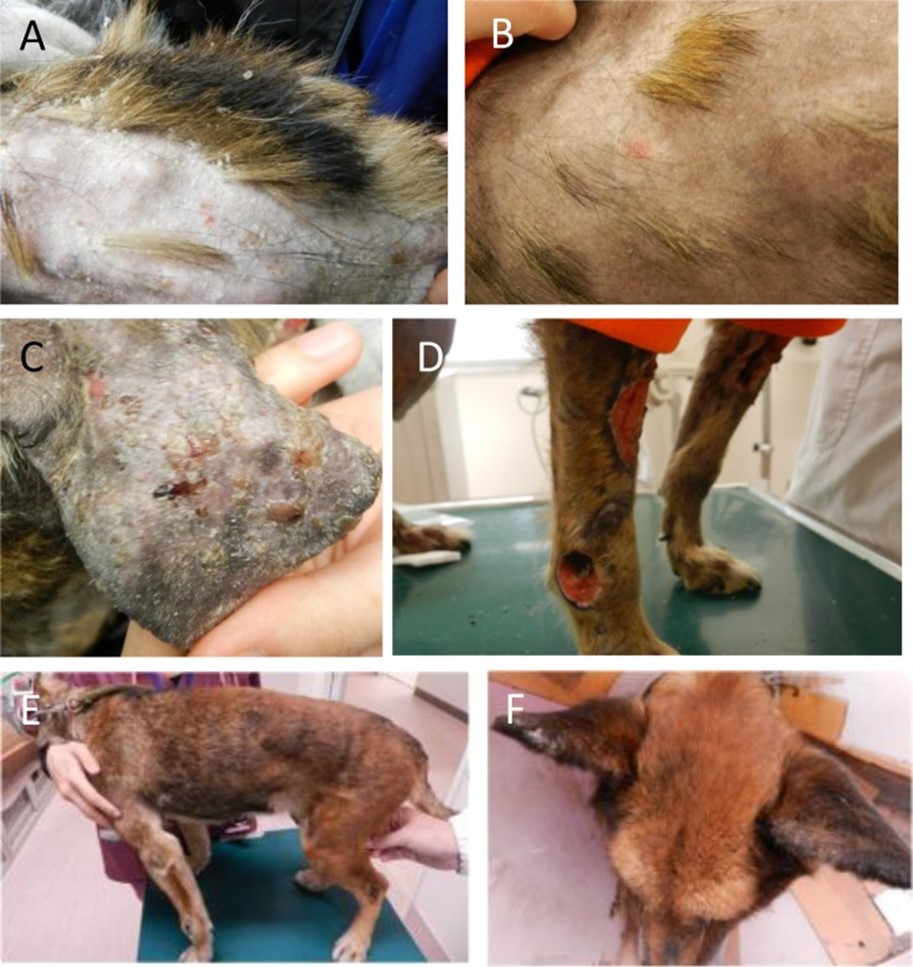
Source: Vet Med Sci. 2013 Oct;75(10):1367-9. doi: 10.1292/jvms.12-0482. A 12-year-old, intact female Satsuma dog was referred to the Kagoshima University Veterinary Teaching Hospital due to skin lesions. Clinical symptoms include erythema, crusts and desquamation on the truck, papules and erosions in the pinnae, and multiple areas of skin necrosis on the right forelimb. Treatment with systemic antibiotics and prednisolone did not improve the observed symptoms. The dog also had progressive anemia. Babesia gibsoni was detected in the blood via PCR during its second visit, and the dog was treated with antiprotozoal agents. Though the cutaneous lesions and anemia improved, skin lesions relapsed after the treatment was discontinued. Histopathological examination of skin biopsies revealed findings suggestive of early leukocytoclastic vasculitis or ischemic vasculopathy… Fig. 1. A–D: Appearance of the dog on initial presentation. A: Alopecia, scales and desquamation in the lumbar area. B: Alopecia and erythema on the trunk. C: Erosion and crusts in the pinnae. D: Cutaneous necrosis on the right forelimb. E, F: Appearance during remission after approximately 2 months of antiprotozoal therapy. E: Alopecia and other skin lesions on the trunk are improved, and the hair color changed from light brown to dark brown. F: Erosion and crusts disappear in the pinnae. Fig. 2. Histopathological sections of affected skin. A: Lower magnification showing perivascular infiltration of inflammatory cells in the middle portion of dermis. Edema and intense extravasation are seen in the superficial dermis. B: Higher magnification of A showing infiltration of neutrophils into the vessel walls and perivascular area, and fibrin deposition adjacent to the blood vessels.
Cortisol: A Short Review
Maigan Espinili Maruquin The well- being of animal can be monitored from stress levels, which is related to the hypothalamic-pituitary-adrenocortical (HPA) axis activity (Möstl and Palme 2002, Salaberger, Millard et al. 2016). Elevation of glucocorticoid concentrations are observed in stressful situations (Salaberger, Millard et al. 2016). https://bluepearlvet.com/medical-articles-for-pet-owners/addisons-disease-in-dogs/ Fig. 01. Location of Adrenal glands which produces glucocorticoids Cortisol is known to play an important role in the interconnected responses on physiological, behavioral, and developmental functions (Bennett and Hayssen 2010). As an adrenal glucocorticoid, acute stress response result to rapid release of glucose from energy stores, suppresses inflammation, and promotes immune cell proliferation (Sapolsky, Romero et al. 2000, Charmandari, Tsigos et al. 2005, Mack and Fokidis 2017). Although measuring cortisol used to require significant disruption in behavior, different non- invasive sample collection methods for cortisol is now being used (Bennett and Hayssen 2010). Cortisol Concentration at Different Tissues In humans, salivary cortisol concentration for measurement is popular due to its straightforwardness and minimal invasive effect, including the fact that the storage is easy (Chen, Cintrón et al. 1992, Wenger-Riggenbach, Boretti et al. 2010). For healthy dogs, cortisol is unbound and passively diffuses from blood to saliva (Kirschbaum, C., et al, 1992) (Vincent and Michell 1992, Cobb, Iskandarani et al. 2016). Within 5 minutes, concentration of free cortisol in saliva and plasma sets at an equilibrium (Tunn, Möllmann et al. 1992, Wenger-Riggenbach, Boretti et al. 2010). Although blood and saliva can provide immediate view of cortisol concentrations, blood collection can be stressful, causing elevated concentrations. On the other hand, saliva absorption materials cause inconsistent results (Dreschel and Granger 2009, Bennett and Hayssen 2010). Cortisol concentrations can be measured for a short time in urine and fecal samples (Bennett and Hayssen 2010). In urine, maximum concentration can be reached at approximately 3 hours (Rooney, Gaines et al. 2007, Bennett and Hayssen 2010) and longer in fecal samples (Bennett and Hayssen 2010). Aside from daily fluctuation of cortisol concentrations in saliva, blood, and urine (van Vonderen, Kooistra et al. 1998), it can also be influenced by any stress of the animal (Kobelt, Hemsworth et al. 2003, Mack and Fokidis 2017). A lot of keratinized tissues contain glucocorticoids (Mack and Fokidis 2017). Concentration in the hair provides a long- term information on glucocorticoid production compared to the concentrations during sample collection (Ouschan, Kuchar et al. 2013). However, based on the effects of hair sampling, analysis of long-term cortisol secretion may be complicated (Mack and Fokidis 2017). Hair may also serve as cortisol storage area, however, validation for every species may be required due to difference in cortisol rhythms, secretions and stress response (Bennett and Hayssen 2010). On the other hand, a study was conducted using dog nails to assess cortisol concentration, wherein nail accumulates cortisol passively from the bloodstream (Mack and Fokidis 2017). Hyperadrenocorticism and Hypoadrenocorticism The chronic overexposure to glucocorticoids like cortisol can result to a complex physical and biochemical changes, referred to as hyperadrenocorticism (HAC) (Ouschan, Kuchar et al. 2013). Clinical signs of an affected dog show polyuria, polydipsia, polyphagia, pot-bellied appearance and typical skin and hair changes (Ouschan, Kuchar et al. 2013) (Feldman EC & Nelson RW, 2004). A tumor in the pituitary gland is usually the cause of this disease, wherein it secretes adrenocorticotrophic hormone (ACTH) and stimulates adrenal glands, resulting to glucocorticoids production (Ouschan, Kuchar et al. 2013). For humans, diagnosis of hypercortisolism and hypocortisolism through the late-night and morning salivary cortisol concentrations is the established screening test (Viardot, Huber et al. 2005, Nieman, Biller et al. 2008, Restituto, Galofré et al. 2008, Wenger-Riggenbach, Boretti et al. 2010). On the other hand, glucocorticoid deficiency is termed to as hypoadrenocorticism (Peterson, Kintzer et al. 1996), wherein, primary adrenal gland failure is the cause of most cases in dogs (Peterson, Kintzer et al. 1996, Gold, Langlois et al. 2016) (Scott-Moncrieff JC, 2010). Due to general and non- specific clinicopathologic signs, it is confused with primary gastrointestinal, renal, or cardiovascular disease. On-time and accurate diagnosis is important because it is a life-threatening disease if it goes without appropriate treatment (Gold, Langlois et al. 2016). References: Addison’s Disease in Dogs Scott-Moncrieff JC. Hypoadrenocorticism. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 7th ed. volume 2. St. Louis, MO: Saunders; 2010:1847–1857. Feldman EC, Nelson RW. Canine hyperadrenocorticism (Cushing′s syndrome). In: Canine and Feline Endocrinology and Reproduction. 3rd edn. St Louis: Saunders, 2004; 252–357. Kirschbaum C, Read GF, Hellhammer D. Assessment of hormones and drugs in saliva in biobehavioral research. Seattle, WA: Hogrefe & Huber; 1992. p. 19–32. Bennett, A. and V. Hayssen (2010). “Measuring cortisol in hair and saliva from dogs: coat color and pigment differences.” Domestic Animal Endocrinology 39(3): 171-180. Charmandari, E., C. Tsigos and G. Chrousos (2005). “ENDOCRINOLOGY OF THE STRESS RESPONSE.” Annual Review of Physiology 67(1): 259-284. Chen, Y. M., N. M. Cintrón and P. A. Whitson (1992). “Long-term storage of salivary cortisol samples at room temperature.” Clin Chem 38(2): 304. Cobb, M. L., K. Iskandarani, V. M. Chinchilli and N. A. Dreschel (2016). “A systematic review and meta-analysis of salivary cortisol measurement in domestic canines.” Domestic Animal Endocrinology 57: 31-42. Dreschel, N. A. and D. A. Granger (2009). “Methods of collection for salivary cortisol measurement in dogs.” Horm Behav 55(1): 163-168. Gold, A. J., D. K. Langlois and K. R. Refsal (2016). “Evaluation of Basal Serum or Plasma Cortisol Concentrations for the Diagnosis of Hypoadrenocorticism in Dogs.” Journal of Veterinary Internal Medicine 30(6): 1798-1805. Kobelt, A. J., P. H. Hemsworth, J. L. Barnett and K. L. Butler (2003). “Sources of sampling variation in saliva cortisol in dogs.” Res Vet Sci 75(2): 157-161. Mack, Z. and H. B. Fokidis (2017). “A novel method for assessing chronic cortisol concentrations in dogs using the nail as a source.” Domestic Animal Endocrinology 59: 53-57. Möstl, E. and R. Palme (2002). “Hormones as indicators of stress.” Domestic Animal Endocrinology 23(1): 67-74. Nieman, L. K., B. M. Biller, J. W. Findling, J. Newell-Price, M. O. Savage, P. M. Stewart and V. M.
