Canine babesiosis
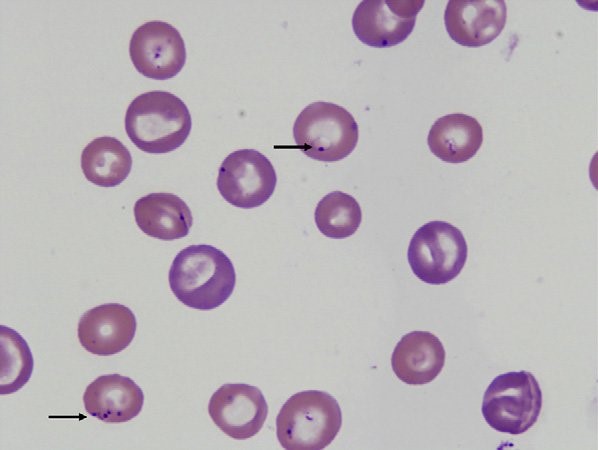
Canine babesiosis Robert Lo, Ph.D, D.V.M Canine babesiosis occurs worldwide and results from infections with a variety of Babesia spp., tick-borne hemoprotozoa. The disease was first described in cattle with hemolytic anemia in 1888 by a Rommanian bacteriologist, Victor Babes (Babes, 1888). Babesiosis is one of the most important tick-borne infectious diseases of domestic and wild mammals and still poses significant diagnostic and therapeutic challenges for veterinary practitioners around the world. More than 100 Babesia spp. were reported in vertebrate hosts (El-Bahnasawy et al., 2002). With the expansion of tick habitats, the spread of parasites into new geographical areas has been an increasing problem worldwide. The Babesia genus belongs to the order Piroplasmida in the phylum Apicomplexa and can be seen as non-pigment forming pear or signet-ring shaped organisms in mammalian erythrocytes. Asexual reproduction occurs in canine erythrocytes while the sexual conjugation and the sporogony stages of their life-cycles occurs in a variety of hard ticks, which can transmit the organism transovarially. Based on their morphology, Babesia are classified into the small Babesia group (trophozoites of 1.0-2.5 µm; including B. gibsoni, B. microti, and B. rodhaini), and the large Babesia group (2.5-5.0 µm; including B. bovis, B. caballi, and B. canis). Etiology and epidemiology Dogs are mainly infected by two species of Babesia: B. canis and B. gibsoni though they can also be infected by several other species of Babesia. Babesia canis has a piriform (teardrop) shape and frequently more than one merozoite is found in a single erythrocyte (Fig. 1). Babesia gibsoni is more pleomorphic (usually oval or signet-ring shapes) (Fig. 2). Fig. 1. Two pear-shaped Babesia canis organisms in an erythrocyte. (Duh et al., 2004) Fig. 2. Babesia gibsoni in erythrocytes in a blood smear stained with modified Wright technique. (Trotta et al., 2009) Babesia canis was further categorized into three subspecies (B. canis canis, B. canis rossi, B. canis vogeli) on the basis of cross-immunity, serological testing, vector specificity and molecular phylogeny (Uilenberg et al., 1989). These three subspecies of babesia are significantly different in their clinical presentation, geographical distribution and vector specificity. With the advent of molecular phylogenetic analysis, in particular that of the 18S rRNA gene, these subspecies are now considered to be separate species (Carret et al., 1999; Lack et al., 2012; Zahler et al., 1998). Recently an unnamed fourth “large” Babesia sp. (coco) has been found in dogs in North Carolina in the US (Birkenheuer et al., 2004) and has caused babesiosis in immunocompromised dogs (Sikorski et al., 2010). The small Babesia are more genetically closer to Theileria spp. than to Babesia spp. based on study of the 185 rRNA gene locus. Babesia canis, transmitted by Dermacentor reicultatus, is the most common pathogen of canine babesiosis in temperate regions of Europe and has been reported sporadically around the world (Solano-Gallego wt al., 2011). Most of clinical cases are reported in spring and autumn, which is associated with the seasonal activity of tick vector (Solano-Gallego et al., 2011; Matijatko et al., 2012). Babesia vogeli, transmitted by Rhipicephalus sanguincus, is a less pathogenic species and is found not only in tropical and subtropical regions but also in colder areas (Cassini et al., 2009). Babesia rossi, transmitted by Haemaphysalis elliptica (syn. Haemaphysalis leachi) (Penzhorn, 2011), is the most virulent species among large Barbesia species and is endemic in southern Africa but has been reported in other regions of eastern and southern Africa (Oyamada et al., 2005). Babesia gibsoni, a virulent parasite in dogs of all ages, is endemic in Asia and occurs sporadically in the rest of the world. Ticks of the complex R. sanguineus may serve as potential vectors for B. gibsoni, at least in Europe, while in Asia, its main distribution range is attributed to transmission by the tick Haemaphysalis longicornis (Hatta et al., 2013; Iwakami et al., 2014). In addition, B. gibsoni can be transmitted by blood exchange when dogs fight (Irwin, 2009). Transmission Babesia spp. are mainly transmitted through tick bites and can infect a wide variety of domestic and wild animals as well as humans (Schnittger et al., 2012). Hard ticks are the main vectors for Babesia spp.; within the tick, Babesia spp. undergo the sexual stage in the tick gut is followed by sporogony in its tissues. The parasite then reaches the tick salivary glands. A blood meal will ultimately transmit the sporozoites from the tick’s salivary gland to their new vertebrate host (Chauvin et al., 2009), where the protozoan undergoes asexual replication (merogony) within the red blood cells. Babesia spp. are transmitted both transstadially and transovarially (Chauvin et al., 2009). Pathogenesis and clinical signs After sporozoites enter the red blood cells, Babesia multiply via repeated binary fission, resulting in up to 16 merozoites. The parasites induce FLP (fibrinogen like proteases) that cause the red blood cells to become sticky, resulting in capillary sludging. Parasitized cells are sequestered in the spleen, and extravascular and intavascular hemolysis occurs. The incubation period following tick transmission is 10-21 days. The clinical picture is similar for all Babesia infections, whether they involve large or small Babesia. Pathogenicity is more severe in young dogs, immunosuppressed dogs, heavily parasitized dogs, and when there is exposure to a virulent strain or concurrent infection (Hunfeld et al., 2008; Matijatko et al., 2012; Schetters et al., 1997; Solano-Gallego L et al., 2008). Infected dogs may exhibit either peracute, acute, or subclinical signs of disease (Freeman et al., 1994; Jacobson, 2006; Wlosniewski et al., 1997). Peracute signs include acute onset of hypotensive shock, vasculitis, extensive tissue damage, hypoxia, and death. Signs of acute disease include fever, lethargy, hemolytic anemia, thrombocytopenia, splenomegaly, lymphadenopathy, icterus, and hemoglobinuria. Less common signs include ascites, peripheral edema, ulcerations, stomatitis, gastroenteritis, CNS signs, acute renal failure, and rhabdomyolysis. Acute infections of virulent strains of B. canis have been associated with induction of the systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) secondary to massive immune
Feline corona virus (FCoV) and Feline infectious peritonitis (FIP)
Feline corona virus (FCoV) and Feline infectious peritonitis (FIP) Andy Pachikerl, Ph.D Introduction: Feline coronavirus (FCoV) is a widely known positive-stranded RNA virus is infecting many cats worldwide (Satoshi, Takehisa and Motonobu 2012). This virus belongs to the species Alphacoronavirus 1 of the genus Alphacoronavirus from within the virus family Coronaviridae (Antoniw and Adams 2013). Alphacoronavirus 1 also includes the canine coronavirus (CCoV), which has been previously reported (link) and the porcine transmissible gastroenteritis coronavirus (TGEV) (Satoshi, Takehisa and Motonobu 2012). There are just two different forms of FCoV namely, the FECV (feline enteric coronavirus) that infects the intestines and FIPV (feline infectious peritonitis virus) that causes the disease feline infectious peritonitis (FIP). Feline coronavirus can typically be found in feces of infected cats and it can be transmitted to healthy cats via coming in contact by means of the fecal-oral route (Hartmann, Feline infectious peritonitis 2005). In environments with multiple cats, the transmission rate is higher as compared to a single-cat environment (Satoshi, Takehisa and Motonobu 2012). The virus is actually symptomless and insignificant until specific mutations occur that can cause complications such as FIP (Satoshi, Takehisa and Motonobu 2012). FIPV is the complication that can be the result of FCoV and causes FIP in cats, for which treatment is generally symptomatic and palliative only. The drug GS-441524 shows promise as an antiviral treatment for FIP, but now it’s still under further research development. Virology Feline enteric coronavirus (FECV) This is caused when the coronavirus becomes prominent in the mature gastrointestinal epithelial cells i.e. enterocytes, brush border, microvilli and villi of the cat (Rottier Peter, et al. 2005). This intestinal infection can show some outward symptoms and is usually chronic. The virus is excreted in the faeces of the symptomless carrier and can solely be detected by polymerase chain reaction (PCR) of faeces or by PCR testing of rectal samples. Cats that are raised in group can infect one another or each other with various strains of the virus. Some cats do show resistance to the virus and are not infected or even become a carrier, while others may become a FECV carrier (Rottier Peter, et al. 2005). Carriers may heal spontaneously but acquired immunity may be short and they may go on to being reinfection. Usually within a few weeks, if they are living in a group with healthy but persistent, excretory carriers; some cats will never heal, and the excretory phase remain permanent. Feline infectious peritonitis (FIPV) and Feline infectious peritonitis (FIP) The virus can further complicate into what is known as FIPV when random errors occur during when the virus infects an enterocyte, causing the virus to mutate from FECV to FIPV (Rottier Peter, et al. 2005). FIPV causes lethal, incurable disease such as: feline infectious peritonitis (FIP). Prior to being domesticated, cats are solitary animals and do not like to share space (i.e. hunting areas, rest areas and defecation sites). Domestic cats living in group therefore have a much higher epidemiological risk of mutation. After this mutation, the FCoV acquires a tropism for macrophages while losing intestinal tropism (Rottier Peter, et al. 2005). Regardless of the source of FIPV and uncertainty about the significance of genetic differences, the relationship between virulence and macrophage/monocyte tropism has been firmly established (Pedersen 2009). While both FIPV and FECV may cause viremia (Gunn-Moore, Bruffydd-Jones and Harbour 1998, Febr, et al. 1996), only FIPV replicates in macrophages and causes the disease (Vennema, et al. 1998, Stoddart and Scott 1989). Complex immune reactions between the virus, antiviral antibodies, and complement cause disseminated vasculitis, which is the hallmark of FIP (Hartmann 2005, Pedersen 2009). In a large group of cats, n, the epidemiological risk of mutation is higher and expressed theoretically as: E = n2 – n. A house hosting 2 cats therefore has a risk of E = 2. When 4 kittens (6 in total) are born into this group, the risk increases from 2 to 30. Overcrowding increases the risk of mutation and conversion from FECV to FIPV, which constitutes a very high-risk factor for the development of FIP cases. FIP has been shown to develop in cats whose immunity is low, such as younger kittens, old cats, immunosuppressed due to viral-FIV (feline immunodeficiency virus) and/or FeLV (Feline leukaemia virus) and stress such as: separation and adoption (Rottier Peter, et al. 2005). The incidence of disease is bimodal, occurring most commonly in cats younger than 18 months and older than 12 years of age. There is a genetic component that contributes to the risk of developing FIP, thus littermates of kittens that have developed FIP are at increased risk. Unfortunately, there is no way to predict, out of a group of FCoV seropositive cats at risk for FIP, which ones are most likely to develop the disease. Infection of macrophages by FIPV is responsible for development of a fatal granulomatous vasculitis, or FIP (see granuloma). Development of FIP depends on two factors: virus mutation and low immunity where virus mutation depends on the rate of mutation of FECV to FIPV and the immune status depends on the age, gene pool and the stress level of the cat. High immune status will be more effective at slowing down the virus (Rottier Peter, et al. 2005). How does FCoV cause FIP? Infections with FCoV are usually limited to the intestinal tract, with very limited viral replication elsewhere. Strains of FCoV causing these infections are referred to as feline enteric coronavirus (or FECV). During infection, and while the virus replicates in the intestine, it undergoes spontaneous mutations. This leads to the development of different strains of the virus, and occasionally a strain may develop that has dramatically altered disease-causing potential – this viral strain is referred to as feline infectious peritonitis virus (FIPV). FIPV strains of FCoV differ from FECV in that they no longer replicate well in the intestine, but rather preferentially infect macrophages – one of the important cells of
Giardia lamblia
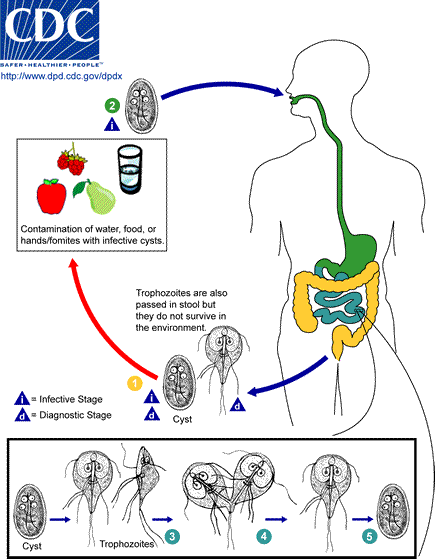
Giardia lamblia LIN, WEN-YANG (WESLEY), Ph.D Giardia lamblia (=G. intestinalis, =G. duodenalis) also called Giardia duodenalis, Giardia intestinalis and pear-shaped flagellate is a common and well-known anaerobic flagellated protozoan parasites colonize in human (or in canine) small intestines and cause diarrhea, stomach pain etc. Its character of parasitic zoonoses make them also infecting other mammalian such as mice, rabbits, birds, reptiles and amphibians. Giardia lamblia prefer to live in cold water like rivers in mountains, cold springs and contaminated pools, which became the major cause of diarrhea in the US. Traveling in the developing world, dinning food without proper cooking, changing diapers etc. would be major risk factors of Giardiasis. The stool tests is the major diagnosis tool. Discovery In 1681, the Dutch scientist Antonie van Leeuwenhoek first found giardia through microscope. In 1915, Giardia lamblia was officially named after scientist Alfred Mathieu Giard who further studied it. In 2010, Andersson et al. has sequenced Giardia’s genome and discovered its ~5000 genes with 11.7 million base pair building blocks. Cellular and physiological properties Giardia is a diplomonad with two nuclei and duplicate organelles follow by four associated flagella and without cytostomes, Golgi apparatus and mitochondria. However, they have a mitochondrial remnant, mitosomes, which take part in the maturation of iron-sulfur proteins rather than ATP synthesis. The life cycle of Giardia lamblia consist of reproductive phase and resting phase that present two different forms: a swimming trophozoite and an infective cyst (Figure 1). Genotyping of Giardia lamblia sub-classified eight genetic assemblages (from A to H). Among eight assemblages, A and B infect are the most dominate assemblages that infect wide range of species including human being. Various species of Giardia exist in different kind of hosts were identified by PCR or genetic tools include G. ardeae and G. psittaci from birds, G. agilis from amphibians, G. microti from voles and G. muris from other vertebrates. Epidemiology In 2013, WHO estimated that there were about 280 million people diagnosed with the Giardia infection around the world. The prevalence rate of Giardia in developed countries was 5% and over 20% among developing countries. In 2018, it present that 15,584 reported cases in 3–7% prevalence of the population in the US, especially high incidence in California, New York, Florida, and Wisconsin. Besides, the highest incidence months of giardiasis would be July, August, and September in the America. Furthermore, 23 of the 31 countries in the Europe had reported total 17,278 confirmed giardiasis cases in 2014. Thus, Giardiasis become one of the top 10 parasite diseases in human beings. Risk factors Travelers to countries where giardiasis is common. Contacting contaminated drinking water, lakes or rivers, animals who have the disease. Giardiasis often happened in the summer because of the higher activities rate in the wilderness. Pathophysiology Giardia exist in rivers, streams, wells and pools. It infect humans or animals by their contacting of contaminated foods, water, contaminated feces and sniffing unclean ground. It is reported that people (or pets) infected with Giardia may have no symptoms, but still spread the disease. Animals such as canines, cows, rodents, beavers, and sheep are also infecting targets of Giardia lamblia. Figure1. The life cycle of Giardia lamblia ( http://www.dpd.cdc.gov/dpdx/images/ParasiteImages/G-L/Giardiasis/Giardia_LifeCycle.gif ) Giardia could transform between cyst form and trophozoite form. It spread and infect host by the cyst form that can remain contagious in cold water up to 3 months. After arriving intestines, Giardia stick to intestinal wall and colonize in the gut by altering its appearance into trophoziote form. Furthermore, they would recover back to cyst form when excreting out with stools. Giardia is spreading through fecal-oral route by animals’ contacting of contaminated water and food. It is one of the most common waterborne outbreaks of diarrhea in the America. Giardia spread between people and animals (Figure 1). When sojourning in the gastrointestinal tract with the trophoziote form, Giardia would engage in inhibiting of brush border enzymes that involved assimilation of disaccharide sugars, altering microvillus’ morphology that cause poor absorption of nutrients and water, triggering apoptosis of intestinal epithelial cells and enhancing intestinal permeability. Eventually, they would cause several clinical symptoms include diarrhea and intestinal malabsorption with or without histological changes. For increasing intestinal permeability, Giardia lamblia would take several dedicated process such as assisting proliferation of crypt cells and using enzymes to degrade proteins on the villi of the brush border. The degraded proteins would likely lead to immunological recruitment and activation of host T lymphocytes on endothelial cells for removing injured cells. In addition, Giardia triggered cell apoptosis by downregulating of the anti-apoptotic Bcl-2 and upregulating of the proapoptotic Bax would facilitate breaking down intestinal barrier and enhancing permeability. They could also execute tactics of consuming all local arginine for decreasing the formation of the gas nitric oxide and protecting its own development. Signs and symptoms The parasitic disease caused from the infection of Giardia, called Giardiasis, also known as beaver fever. A 10% of Giardiasis can be temperate and showed no sign, which recovery by host’s immune function without extra treatments. Nevertheless, 90% infected hosts show apparent symptoms 2 days after infection and last 1 to 6 weeks. Giardia would make atrophy in the small intestinal villi that would lead to foul- smelling diarrhea and cause dehydration accompanied with malnutrition. Diarrhea is the most common and dominant sign (in both humans and animals) accompanied by other symptoms include excess gas, abdominal cramps, abdominal pain, weight loss, nausea, vomiting, bloody feces and fever. However, only about 15% of infected hosts would occur fever. Most canines would be less active instead of exhibiting fever. Lesser common cases shows signs beyond intestinal system as itchy skin, hives and swelling of the eyes and joints. Medication should be administrated as soon as possible. Chronic diarrhea could last for weeks or months if untreated. Symptoms such as post-infectious irritable bowel syndrome, lactose intolerance and food allergies may occur and remain even if Giardia were dealt with medication. Diagnosis Detecting antigens in stool specimens, which
Breed-related disease: Himalayan

John K. Rosembert The Himalayan cat is a medium-sized cat very similar in appearance to the Persian cat but distinguished by the points on the cats’ extremities (the facial mask, feet, ears, and tail) which results in a Persian-type cat with the coloring and deep blue eyes of the Siamese-patterned cat. The ideal Himalayan is a strong cat with excellent boning and musculature, a well-balanced cat, giving the impression of robust power. As with their Persian cousins, the Himalayan cat has two facial variations : traditional / doll-faced, or peke-faced (named after the Pekingese dog with squashed-looking features). The Himalayans make great indoor pets. They possess the best characteristics from the Siamese and the Persian. Their activity levels lay between that of the Siamese and Persian, so they’re equally happy to play as they are to relax, making them great family pets They are loyal and affectionate cats who require lots of attention and love but tend to play favorites among their owners. They are very social, sweet and intelligent, and have been known to be quite talkative. Here we bring you the most common Himalayan Cat Diseases & Conditions. Polycystic kidney disease. PKD is a condition that is inherited and symptoms can start to show at a young age. Polycystic Kidney Disease causes cysts of fluid to form in the kidneys, obstructing them from functioning properly. It can cause chronic renal failure if not detected . Look for symptoms like poor appetite, vomiting, drinking excessively, frequent urination, lethargy and depression. Breathing issues. Peke-faced cats have a compacted snout and airway and as a result, it may suffer shortness of breath or noisy breathing. Cherry eye . is a condition affecting the eye, causing the third eyelid to well and cause irritation It generally appears as a red mass (hence the name cherry) on the corner of the cat’s eye. It is treated with surgery. Progressive retinal atrophy. Refers to a family of eye conditions which cause the retina’s gradual deterioration. Night vision is lost in the early stages of the disease, and day vision is lost as the disease progresses. Many cats adapt to the loss of vision well, as long as their environment stays the same. Entropion. It is a condition that can occur in Persians and causes the eyelid to roll inwards, which can lead to irritation or injury of the eyeball. Signs include rubbing or scratching around the eye area. It can be treated surgically if necessary. Primary seborrhea. is a skin condition in which the skin becomes greasy, scaly and smelly due to the overproduction of skin cells. Sources: http://www.vetstreet.com/cats/himalayan#1_ugw20zmq https://bowwowinsurance.com.au/cats/cat-breeds/himalayan-cat/ Photo credit: https://www.shutterstock.com/ search / himalayan + cat https://www.wwwallaboutcats.com/best-food-for-himalayan-cats
Breed-related disease: Siberian Husky

John K. Rosembert The Siberian Husky is a medium-sized working dog breed with a thick coat that comes in a multitude of colors and markings. Their blue or multi-colored eyes and striking facial masks only add to the appeal of this breed, which originated in Siberia. It is a breed that combines power, speed, and endurance. They are moderately compact, slightly longer than they are tall, and of definite Northern heritage. They are quick and light on their feet, with a smooth and effortless stride exhibiting both good reach and drive. Their expression is often keen but friendly, interested, and sometimes even mischievous. The Siberian Husky breed has an average lifespan of 12 to 14 years and is an ideal pet choice for lots of different people, including families. However, purebred Huskies do have several canine health problems that prospective owners should consider. As with all animals, it’s important to be aware of the common health concerns that plague Siberian Huskies since many of the problems can be expensive and time-consuming to treat. If you’re thinking of adopting a Husky, learn about their common health problems here so you can make an informed decision about the requirements of the dog breed. Here’s a list of the most common health problems for Siberian Husky: Cataracts: which is cloudiness in the lens of the eye leading to blurred vision, which ranges from partial to complete opacity. When the lens becomes cloudy it can prevent light from passing to the retina, contributing to vision loss. It is one of the most common health problems for Siberian Huskies, affecting about 10% of the breed, it will typically develop within 6 to 12 months of a Husky’s life and can, unfortunately, lead to blindness later on. Because of this, it’s important to have your dog’s eyes checked by a vet regularly. Progressive Retinal Atrophy (PRA): is also sometimes known as progressive rod and cone degeneration (PRCD), is a group of degenerative eye disorders in dogs that lead to blindness in both eyes.It is another common eye problem for Siberian Huskies. With PRA, the retina of a dog’s eye starts to deteriorate. Both cataracts and progressive retinal atrophy are considered to be costly health issues, so early detection is vital. Corneal Dystrophy: Corneal dystrophy is a hereditary disease that affects the cornea of Siberian Huskies. If your Husky is suffering from this condition, you will notice small white dots in his cornea. Huskies with this health issue may experience opaqueness or at times, hazy vision. Sadly, there is currently no known treatment for correcting corneal dystrophy. Uveodermatologic Syndrome: Uveodermatologic syndrome is another common eye disease with the Siberian Husky, although this condition also affects the skin as well as the nervous system. It’s important to keep in mind that the skin reaction to this syndrome is only cosmetic. However, its effects on the eyes can cause blindness in severe cases. Uveodermatologic syndrome is difficult to detect, but the first signs will usually be in a Husky’s eyes. Many dogs may show redness in their eyes as well as impaired vision. Hip Dysplasia: is an abnormal formation of the hip socket that, in its more severe form, can eventually cause crippling lameness and painful arthritis of the joints, it is one of the most concerning health issues for any dog owner to worry about, it is very common with Siberian Huskies. Follicular Dysplasia: is a genetic disease causing alopecia, or hair loss. It is caused by hair follicles that are malfunctioning due to structural abnormality. This condition affects Huskies between 3 and 4 months of age and can result in abnormal hair growth, canine hair loss, or patchy, infectious skin. Siberian Huskies have a high risk of follicular dysplasia and unfortunately, there is currently no treatment. To better manage the disease, some vets will recommend that pet owners use specific shampoos, antimicrobials, and topical applications as needed. Zinc Deficiency: Just like humans, dogs need a sufficient amount of zinc in their bodies in order to remain at optimum health. When Siberian Huskies experience a zinc deficiency, they might suffer from hair loss on their feet, elbows, or eye, chin, and lip areas. Zinc supplements may help alleviate symptoms, but a vet should be consulted before adding any to your pet’s diet to avoid an overdose. Hypothyroidism: is a common health problem in Huskies that relates to an abnormal amount of secretion of the thyroid gland. If your Siberian Husky has this condition, you may notice that he’s gained weight, although he is eating less than normal. You might also notice fur loss or even bald spots located on his coat. Other symptoms include lethargy and increased sleep. Sources: https://dogtime.com/dog-breeds/siberian-husky#/slide/1 https://canna-pet.com/siberian-husky-health-problems-issues/ Photo credit: http://www.opsopet.com/product/siberian-husky-dog/ https://www.clicktreatplay.com/know-dog-siberian-husky/
Breed-related disease: Siamese cat

John K. Rosembert Siamese, popular short-haired breed of domestic cat originally from Thailand, a country whose official name was Siam until 1939. The Siamese is a lithe long-bodied cat with slim legs and a long slim tail. It has a long wedge-shaped head and blue eyes. They have a distinctive “pointed” coat: a light-colored background with darker points on the ears, mask, legs, and tail in the seal, lilac, chocolate and blue. Other point colors include tabby, red, cream , silver and smoke. The Siamese was first exported from Siam to the United States in 1878 and the United Kingdom in 1884. By 1902 the first cat fanciers club devoted to the Siamese cat had been established in the United Kingdom, and by 1906 the Cat Fanciers’ Association had officially recognized the breed. The Siamese come in two types: “show and traditional”. The show Siamese is a work of modern art, all lines, and angles. He has a tubular body on long legs, a wedge-shaped head with large, triangular ears, and a long tail. The traditional Siamese, also known as the apple-headed Siamese, has a rounded head and chunky body. Both types have bright blue eyes that demand worship due to all cats. Whether you choose a show Siamese or a traditional Siamese, they should share the same wonderful personality. They are endlessly curious but inclined to be smart and demanding. This is a cat who has a passion for his people and will involve himself in everything they are doing. Here are some of the common health issues to watch out for in Siamese Progressive retinal atrophy (PRA): Siamese cats are prone to this genetic eye problem which leads to progressive blindness. For diagnosis and to help your cat lead as normal a life as possible with PRA, input is needed from a veterinary ophthalmologist. Systemic amyloidosis: This is where protein builds up in various organs, stopping them from working properly, typically resulting in severe liver damage and / or kidney failure. This is a fatal disease. Mediastinal lymphoma: This form of cancer causes a buildup of fluid around the lungs. From chemo and steroids through to possible surgery, a vet should be able to advise you on the best way forward. Asthma: Siamese are one of the cat breeds most susceptible to this disease of the airway. Treatment options include anti-inflammatory drugs and bronchodilator therapy. Hip dysplasia: Siamese can be predisposed to this form of lameness of the hind leg, caused by abnormal development of the hip joint. It can’t be cured-but it can often be controlled. Vestibular Disease: Some Siamese cats develop vestibular disease. This is a genetic problem having to do with the inner ear, specifically the nerves serving the ear. Cats with vestibular disease will display symptoms consistent with a loss of balance, such as head tilting. Sources: https://www.britannica.com/animal/Siamese-breed-of-cat https://www.everypaw.com/all-things-pet/siamese-cat-breed-info-and-health-advice Photo credit: http://www.vetstreet.com/cats/siamese#0_ryz4d6cf https://www.catster.com/topic/offbeat
Feline Leukemia Virus (FeLV): A Constant Threat to Our Cat Companion
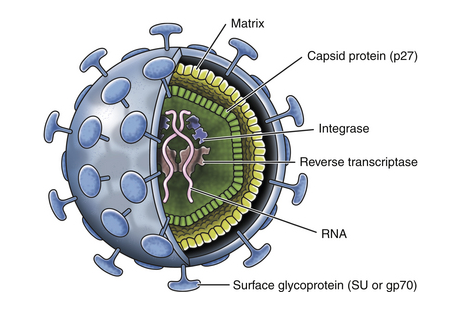
[vc_row][vc_column][vc_column_text] Feline Leukemia Virus (FeLV): A Constant Threat to Our Cat Companion Maigan Espinili Maruquin It was believed that the Feline Leukemia Virus (FeLV) is the one responsible in most disease- related deaths in cats. It was Jarrett, et al., 1964 who first identified FeLV as a causative agent of the viral infection of cats more than 40 years ago by electron microscopy (EM). However, the prevalence of FeLV as the disease- causing agent in cats has declined, and so is the death rate caused by the infection. Despite FeLV being a threat in the life expectancy of the cats, owners still choose to provide the proper treatment for their cats and proper care, leaving FeLV-infected cats live for many years with good quality of life (Hartmann 2012). Structure and Replication (https://veteriankey.com/feline-leukemia-virus-infection/) Fig. 01. The structure of FeLV containing two identical strands of RNA, reverse transcriptase, integrase, and protease inside the capsid protein (p27), surrounded by a matrix and all enclosed by the envelope containing gp70 glycoprotein and the transmembrane protein p15E (https://veteriankey.com/feline-leukemia-virus-infection/). FeLV is approximately 8.4kb in length. It belongs to the genus Gammaretrovirus. Retroviruses have three- layered structure and RNA (two copies of single-stranded RNA), which makes the genetic material, is in the innermost- layer; together with the essential enzymes for its viral activities (including integrase, reverse transcriptase and protease) and nucleocapsid protein. The capsid protein in the middle layer surrounds the genome. And, the outer layer is the envelope from which glycoprotein ‘spikes’ project (Westman, Malik et al. 2019). The envelope spikes are responsible for the attachment of the virus to the target cell surface receptors which also represents an essential target for the host immune response. During replication, RNA is being reverse transcribed into DNA through the enzyme reverse transcriptase. This interrupts the normal cellular flow of genetic information, the Central Dogma, making this enzyme the target of many anti- viral drugs. The synthesized DNA from the RNA integrates into the genome of the target cell as a provirus, which is a required component for the viral replication, assisted by a second viral enzyme, the ‘integrase’. This provirus remains in the genome of the cell and upon cellular division, the provirus is expressed, leading to the production of progeny virions and virus shedding (Lavialle, Cornelis et al. 2013, Willett and Hosie 2013, Chiu, Hoover et al. 2018, Westman, Malik et al. 2019). FeLV Infection On a previous research, there were three important observations following FeLV Infection: (a) some cats can eliminate the virus before it progresses local replication after enough time and appropriate immune response; (b) some cats become persistently viraemic; (c) some cats are viraemic before immunity responds to eliminate the transient viraemia after 2–16 weeks, but not before a latent infection is established as DNA provirus (Westman, Malik et al. 2019). Antigen-negative, provirus positive cats are considered FeLV carriers. This was after cats infected with FeLV were found to remain provirus-positive. Following reactivation, they can act as a source of infection. As FeLV provirus is integrated into the cat’s genome, it is unlikely to be fully cleared over time and possibly in a transcriptionally silent (latent) state. Antigen-negative, provirus-positive cats do not shed the virus, but reactivation is possible (Torres, O’Halloran et al. 2008, Hartmann 2012). FeLV undergoes different stages of infection. On abortive infection, virus starts initial replication but an effective immune response may terminate the viral replication and avoid becoming viraemic by eliminating the FeLV-infected cells (Hofmann-Lehmann, Cattori et al. 2008, Torres, O’Halloran et al. 2008, Hartmann 2012)(Torres, Mathiason et al. 2005). In regressive infection, effective immune response contains the replication of the virus prior to or shortly after bone marrow infection (Hartmann 2012) despite retaining a low level of FeLV-infected cells in circulation and tissues. In some cases, infected cells are also eliminated and undergo abortive infection (Torres, Mathiason et al. 2005). Mainly, virus shed in saliva however, in this infection, viremia is terminated within weeks or months. However, virus undergoes latency since it is not completely eliminated, harboring viral DNA in circulation, and integrating the proviral DNA in the bone marrow stem cells and lymphoid tissues. The proviral DNA is not translated into proteins making it non- infectious. The cats are considered ‘protected’ from the development of viraemia and thus disease, but they remain infected. Under latent infection, viral replication is delayed. Therefore, these regressively infected cats are not infectious to others but the infection could be reactivated when antibody production decreases (Torres, Mathiason et al. 2005, Hofmann-Lehmann, Cattori et al. 2008, Torres, O’Halloran et al. 2008, Hartmann 2012). For the progressive infection, the infection is not contained early, resulting to extensive viral replication. They remain positive after 16 weeks of infection. This makes the cats persistently viraemic and infectious to other cats. They develop FeLV- related diseases, and most of them die within a few years. On the other hand, it is focal or atypical infection if there’s a persistent atypical local viral replication (e.g., in mammary glands, bladder, eyes). This leads to an irregular production of antigen causing alternate results of positive and negative (Hartmann 2012). Clinical Signs/ Pathogenesis There are two possible results following the first 4 weeks FeLV exposure of the host: (a) failure to contain the viral replication; and (b) successful immune response of the host against the virus (Rojko, Hoover et al. 1982, Hoover and Mullins 1991, Torres, Mathiason et al. 2005). After a long asymptomatic phase, cats can develop clinical signs including tumors, hematopoietic disorders, neurologic disorders, immunodeficiency, immune-mediated diseases, stomatitis, immunosuppression, hematologic disorders, immune-mediated diseases, and other syndromes (including neuropathy, reproductive disorders, fading kitten syndrome). This is determined by a combination of viral and host factors (Hartmann 2012). Mostly, tumors in cats are associated with FeLV, commonly lymphoma and leukemia, less often other hematopoietic tumors and rarely other malignancies (including neuroblastoma, osteochondroma, and others). FeLV vaccination resulted to a major decrease of FeLV infection in the overall
Rapid Antimicrobial Susceptibility Testing to Combat Resistance
Table of Contents 1. Introduction: Defining Antimicrobial Susceptibility Testing (AST) Antimicrobial Susceptibility Testing (AST) is a crucial diagnostic procedure used to guide the treatment of infectious diseases. It provides evidence-based data that allow clinicians and veterinarians to identify the most effective antibiotics for treating specific bacterial infections while minimizing the risk of antimicrobial resistance (AMR). 1.1 Purpose in Clinical and Veterinary Diagnostics AST is an in vitro laboratory method used to determine which antibiotics are effective at inhibiting the growth of a given bacterial isolate. In clinical microbiology, it functions as a vital extension of the diagnostic process, translating laboratory findings into actionable treatment decisions. The fundamental objectives of AST are threefold: Confirm Susceptibility: To verify that a bacterial isolate is sensitive to the chosen empirical antimicrobial agents. Detect Resistance: To identify emerging or established resistance mechanisms within the isolate. Guide Therapy: To provide clinicians with data that support targeted, rational antimicrobial selection. Although most applications are in human medicine, the relevance of AST extends to veterinary and agricultural diagnostics, where inappropriate or preventive antibiotic use in food and animal industries accelerates the development of resistance. Incorporating AST into these sectors is therefore essential for achieving a One Health approach that integrates human, animal, and environmental health management. 1.2 Role in Identifying the Most Effective Antibiotic AST ensures that patients receive the most appropriate and targeted antibiotic therapy. The process combines quantitative and qualitative assessments of bacterial growth inhibition, allowing for the identification of the most suitable antimicrobial agent. Minimum Inhibitory Concentration (MIC):A primary output of AST is the Minimum Inhibitory Concentration (MIC), defined as the lowest concentration of an antibiotic required to inhibit visible bacterial growth in vitro. Determining Efficacy:MIC values are interpreted to determine whether the bacterial isolate is susceptible, intermediate, or resistant to a given antibiotic. Standardization and Global Guidelines:The Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) establish standardized interpretive breakpoints that guide laboratory and clinical decisions globally. Integration for Complete Diagnosis:The diagnostic value of AST is maximized when combined with accurate bacterial identification. This integration enables physicians and veterinarians to administer the narrowest effective antibiotic for the identified pathogen, optimizing therapeutic outcomes and minimizing ecological impact. 1.3 Supporting Antimicrobial Stewardship and Reducing Misuse AST forms the cornerstone of antimicrobial stewardship, the coordinated effort to preserve antibiotic efficacy by promoting rational use. The global threat of antibiotic resistance, which currently contributes to an estimated 700,000 deaths annually, underscores the urgency of this practice. Reducing Broad-Spectrum Dependence:In many clinical scenarios, delays in diagnostic confirmation lead clinicians to initiate broad-spectrum antibiotics empirically. This practice, while often necessary, fosters selective pressure that accelerates resistance. Enabling Rapid, Targeted Treatment:Improvements in AST turnaround time and the adoption of rapid testing methods allow faster initiation of effective targeted therapy, reducing unnecessary broad-spectrum exposure. Improving Clinical Outcomes:Timely susceptibility results enable healthcare professionals to transition from empirical to pathogen-directed therapy. This precision approach improves patient recovery, reduces adverse effects, and contributes to long-term containment of resistance. 2. Principles and Methods of Antimicrobial Susceptibility Testing (AST) Antimicrobial Susceptibility Testing (AST) encompasses a range of laboratory methods designed to determine the ability of bacteria to grow in the presence of specific antimicrobial agents. These techniques are broadly divided into phenotypic methods, which measure the observable inhibition of bacterial growth, and genotypic methods, which detect genetic determinants of antimicrobial resistance. 2.1 Phenotypic Methods Phenotypic testing remains the gold standard for AST in clinical and veterinary microbiology. These methods directly assess bacterial growth inhibition and provide either qualitative or quantitative results based on visible morphological changes. Disk Diffusion (Kirby–Bauer Test) The disk diffusion method, commonly known as the Kirby–Bauer test, is one of the most widely used AST procedures worldwide due to its convenience, low cost, and standardized interpretive criteria. Principle and Procedure:Sterile paper disks impregnated with fixed concentrations of antibiotics are placed on the surface of an agar plate uniformly inoculated with the bacterial isolate. Following incubation (typically 16–24 hours at 35 °C), bacterial growth is inhibited around the disk, forming a zone of inhibition. Interpretation:The diameter of the inhibition zone is measured and compared with reference standards provided by the Clinical and Laboratory Standards Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing (EUCAST). The isolate is categorized as susceptible, intermediate, or resistant based on these standardized breakpoints. Advantages and Limitations:The disk diffusion test allows simultaneous testing of multiple antibiotics but provides qualitative results only, as it does not yield an exact Minimum Inhibitory Concentration (MIC). Broth Dilution (Macro and Micro Methods) Broth dilution techniques determine the MIC, defined as the lowest antibiotic concentration that inhibits visible bacterial growth. Macrobroth (Tube) Dilution:This traditional method involves preparing serial two-fold dilutions of antibiotics in test tubes containing a liquid growth medium. After inoculation and incubation at 35 °C, tubes are examined for turbidity. The lowest concentration preventing visible growth represents the MIC. While reliable, this approach is labor-intensive and time-consuming, limiting its routine clinical use. Broth Microdilution:This method miniaturizes the macrobroth technique using 96-well microtiter plates, allowing the simultaneous testing of multiple antibiotics and bacterial isolates. Each well contains a defined concentration of antibiotic and a standardized inoculum. After incubation, bacterial growth is assessed visually or via automated readers. Automation: Broth microdilution forms the foundation of most automated AST systems, which have been in routine diagnostic use since the 1980s. Systems such as VITEK® 2, BD Phoenix™, Sensititre™, and MicroScan WalkAway® automate sample handling, incubation, and interpretation, improving standardization and efficiency. Turnaround Time: Although miniaturized, conventional broth microdilution typically requires similar incubation times to macrobroth methods (16–24 hours). E-test (Gradient Diffusion Method) The E-test provides a semi-quantitative estimate of the MIC by combining diffusion and dilution principles. Principle and Procedure:A plastic strip impregnated with a continuous antibiotic gradient is placed on an agar plate inoculated with the test organism. After 18–24 hours of incubation, an elliptical inhibition zone forms around the strip. Interpretation:The MIC is read directly from the point on the scale where the inhibition
Canine Parvovirus
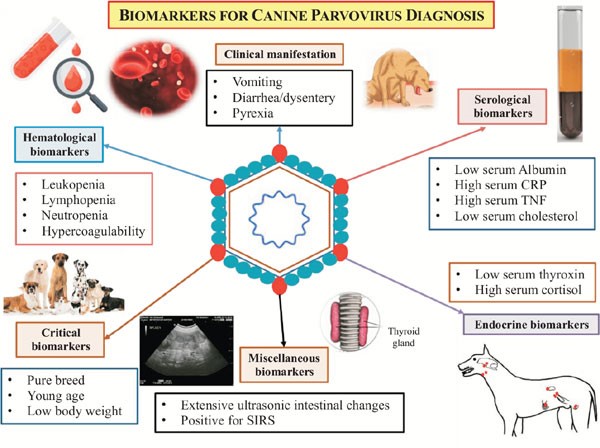
Canine Parvovirus CHINESE EDITION IS WRITTEN BY DR. WANG, SHIH-HAO / ENGLISH EDITION IS TRANSLATED AND EDITED BY DR. LIN, WEN-YANG (WESLEY) Abstract The canine parvovirus (CPV) is a common, acute, high morbidity and high morality virus that mainly infect canine population. This virus possess highly survival rate for 5 weeks in the natural environment. It is highly contagious and easily transmitting among canine population by the fecal-oral route through contacting contaminated feces. CPV usually attack digestive system. Sometimes it may induce myocarditis among canine and cause sudden death. All ages, sexes and breeds of dogs could be susceptible to CPV, especially puppies. Clinical sighs of infected dogs may include fever, lethargy, continuous vomiting, continuous diarrhea, stinky viscous diarrhea with blood, dehydration and abdominal pain etc. Canine show signs of the disease would usually die within 3 to 5 days. There are no specific drugs for curing CPV until now. Supportive care such as consuming water-electrolyte fluid is the only present solution to maintain physiological function and relieve symptoms. The infected canine should have medical care as soon as possible; otherwise, more severe conditions like acute dehydration, hypovolemic shock, bacterial infections and death will occur. Infection prevention measures include environmental disinfection and routine vaccines. Pathogens The canine parvovirus (CPV) is an ssDNA virus, which belongs to the species carnivore protoparvovirus 1 within the genus protoparvovirus in the family parvovirus (parvoviridae). CPV is 98% identical to feline panleukopenia virus (FPLV) with variant in six coding nucleotide of structural proteins VP2: 3025, 3065, 3094, 3753, 4477, 4498 that makes CPV-2 infect canine host instead of replicating in cats. Two types of canine parvovirus were discovered – canine minute virus (CPV1) and CPV2, both can attack canine population and canidae family such as raccoons, wolves and foxes. Canine parvovirus may be susceptible to cats without pathogenic, and it is an inapparent infection. CPV2 could stably survive in feces for 5 months with ideal condition. Furthermore, CPV-2a, CPV-2b and CPV-2c type viruses have been isolated and sequenced from animals. Other than targeting on canine, large cats are susceptible to CPV-2a, CPV-2b. CPV-2c type viruses have high prevalence on infecting leopard cats. Figure 1. Model of CPV evolution showing VP2 amino acid differences between each virus and indicating the virus host ranges. (Karla M. Stucker, Virus Evolution In A Novel Host: Studies Of Host Adaptation By Canine Parvovirus, Published in 2010) Epidemiology In 1978, a novel infectious canine disease was firstly occurring in the east coast of America. Within 12 months, scientists identified CPV-2 as the aetiological key of severe symptoms among canine. Due to characters of highly contagious and potential environmental resistance, CPV-2 spread swiftly over entire USA, European countries, Australia and Asia. In 1978, canine parvovirus also invade among canine in Taiwan. Therefore, CPV caused large scale of canine death at the early stage of pandemic. By the establishment and development of CPV vaccine, global wide spreading of CPV has been rarely happen today. However, canine parvovirus still widely exists in domestic dogs and wild canidae. It became one of the canine endemic disease. Pathogenesis Incubation period of CPV-2 lasts 4 to 5 days. The virus mostly attacks rapidly dividing cells especially lymphopoietic tissues, the bone marrow, crypt epithelia of the jejunum, ileum and (in young dogs under 4 weeks old) myocardial cells. Rottweilers, black Labrador Retrievers, Doberman Pinschers, and American Pit Bull Terriers are more susceptible than other species; once they are infected, would suffer severer conditions. Besides, CPV-2 take the major place to affect canine and wild canids. After entering into hosts’ body, CPV-2 firstly replicates in oropharynx lymphoid tissues, mesenteric lymph nodes and thymus gland, then spreading to other lymph nodes, lung, liver, kidney and rapidly dividing tissues (e.g. bone marrow, intestinal epithelial cell and myocardial cell) by the blood stream. 4 to 5 days after, clinical sighs like diarrhea, vomiting, lymphopenia, anorexia, depression, dehydration, hypothermia, thrombocytopenia and neutropenia would appear. Severe dehydration and hypovolemic shock may happen due to lose large amount of fluid and protein by vomiting and diarrhea. Transmission Fecal-oral route is the main transmission pathway of CPV-2. Large amount of virus would be detected in feces of infected canine within 1 to 2 weeks of acute phase. An infected pregnant canine could transmit virus to fetus through placenta. Fomites include contaminated shoes, cages, food bowls and other utensils could serve as CPV transmitting objects also. Clinical forms There are four clinical forms according to distinct signs and lesions: enteric, myocardial, systemic infection and inapparent Infection. A. Enteric form : It is known that CPV-2 caused enteritis symptoms. This form infect host with low virus titers (around 100 TCID50). Symptoms in initial stage are sopor, loss of appetite, acute diarrhea, vomiting, dehydration, slight elevated body temperature, frailty and acting like in extreme pain. Severity of illness vary according to the age of canine, healthy condition, infectious dose of the virus, and other pathogens in intestine and so on. Typical signs of CPV induced enteritis and its course include loss of appetite, sopor, fever (39.5℃-41.5℃) within 48 hours follow vomiting. 6 to 24 hour after vomiting follow watery stool in yellow or white color, mucus stool or bloody stool with stench in severe cases. Due to consistent diarrhea and vomiting, dogs suffer worsen dehydrated condition. Common clinical pathologic examination consist assessing dehydrated condition and significant decreasing of white blood cell of dogs (400 to 3000 /μL). B. Myocardial form: This form only appear in puppies around 3 to 12 weeks of age. Major cases show pups’ age under 8 weeks. Mortality rate is extremely high with myocardial form (almost up to 100%). Clinical signs include irregular breathing, cardiac arrhythmia. Collapse, hard breathing may happen to acute cases follow death within 30 minutes. Most cases would die within 2 days. The subacute form would also die from hypoplastic heart syndrome within 60 days. Nevertheless female adult canine acquire antibodies against myocardial form by vaccination or infection, puppies may
Peritonitis in Dogs: Causes, Diagnosis, and Treatment Insights

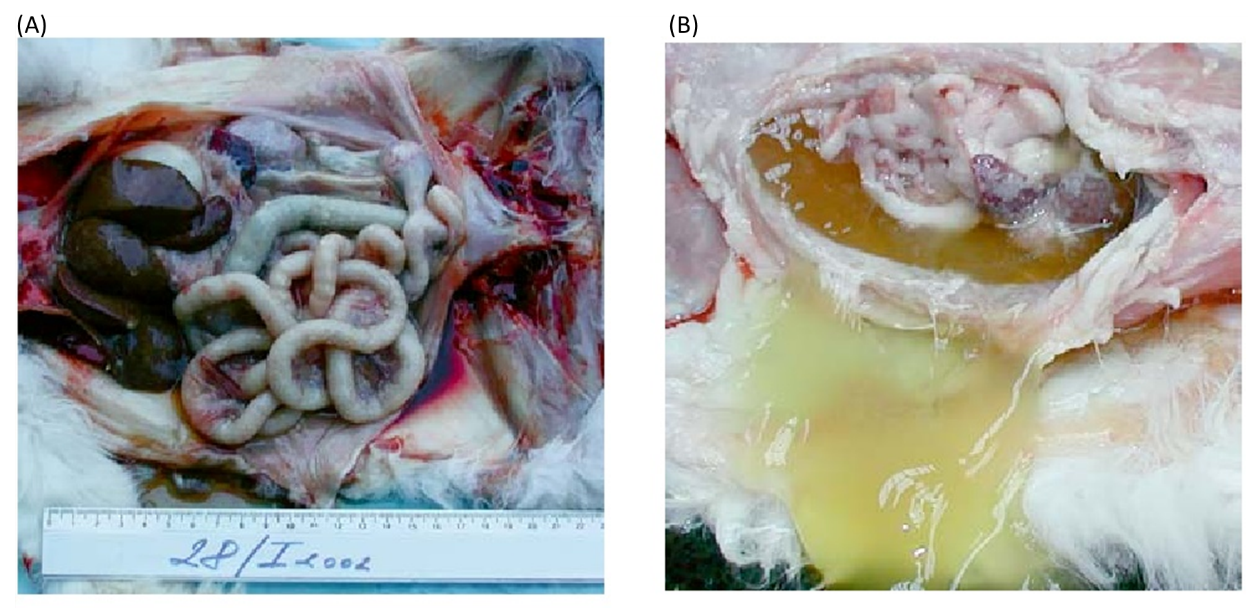
Table of Contents 1. Introduction to Peritonitis and Septic Peritonitis (SP) Definition of Peritonitis Peritonitis in dogs refers to the inflammation of the peritoneum, the thin serous membrane that lines the abdominal cavity and envelops the visceral organs. When this inflammation is accompanied by microbial contamination, the condition progresses to septic peritonitis (SP), a complex, rapidly progressive, and life-threatening disease. SP represents a convergence of local peritoneal inflammation and systemic infectious insult, frequently culminating in sepsis or septic shock without timely intervention. Relevance Across Mammalian Species The pathophysiological patterns of septic peritonitis exhibit strong parallels across mammalian species. Data derived from canine, feline, and human literature suggest similar clinical trajectories and diagnostic challenges: Canine–Feline Parallels:Evidence indicates that clinicopathologic abnormalities and outcomes in feline SP mirror those documented in dogs, reinforcing the cross-species applicability of diagnostic and therapeutic strategies. Human Literature as a Clinical Framework:Human medicine, with its extensive sepsis research, provides a valuable framework for veterinary clinicians. The early adoption of Procalcitonin (PCT) as a biomarker in dogs reflects its well-established role in human sepsis diagnosis.The central mechanism, an imbalance between pro-inflammatory and anti-inflammatory immune responses, is widely corroborated by human critical care studies and applies equally to canine SP. Peritonitis Arises Most Commonly from Infection and Perforation Microbial contamination of the peritoneal cavity most frequently results from gastrointestinal perforation, loss of mucosal integrity, or traumatic breach of sterile abdominal compartments. Microbial Etiology Gram-negative organisms, particularly Escherichia coli, predominate in septic abdominal infections: In one study of dogs with septic peritonitis: 39 percent of abdominal cultures yielded only gram-negative bacteria, 28 percent yielded only gram-positive organisms, and 33 percent demonstrated mixed gram-negative and gram-positive infections. Common Sources of Contamination Septic peritonitis is usually linked to a definable intra-abdominal lesion or event: Gastrointestinal leakage, including dehiscence of enterotomy or enterectomy sites, remains one of the most frequent causes. NSAID-induced perforation: Meloxicam-associated colonic perforation is documented, marked by full-thickness ulceration and underlying vascular thrombosis, culminating in diffuse septic peritonitis. Parasitic migration and necrosis: Aberrant migration of Spirocerca lupi may induce acute mesenteric ischemia-like lesions, leading to segmental necrosis, infarction, and SP. In one case series, all affected dogs were ultimately diagnosed with septic peritonitis. These pathways highlight the diversity of initiating events while reinforcing the consistent pathogenic mechanism, the introduction of bacteria or fungi into a previously sterile compartment. Importance of Rapid Recognition and Progression Toward Shock Septic peritonitis is characterized by abrupt clinical deterioration. Early recognition and decisive intervention are central to improving survival. Life-Threatening Pathophysiology Sepsis is defined as a life-threatening organ dysfunction arising from a dysregulated host response to infection. SP is a major precipitating cause of sepsis in veterinary practice. Rapid Clinical Decline The literature consistently stresses the importance of early diagnosis: Delayed detection directly decreases survival, as timely intervention is essential for controlling contamination and stabilizing systemic physiology. Dogs with bacterial SP frequently fulfill Systemic Inflammatory Response Syndrome (SIRS) criteria due to their pronounced inflammatory cascade. Progression to Septic Shock SP-associated sepsis may escalate to septic shock, defined by: Persistent arterial hypotension despite aggressive fluid resuscitation Requirement for vasopressor therapy to achieve adequate perfusion pressure In one study, 25 percent of dogs with bacterial sepsis progressed to septic shock requiring vasopressors, reflecting the severe systemic compromise associated with SP. Mortality and Organ Dysfunction Survival outcomes correlate strongly with: The number of organ systems affected, and The severity of organ dysfunction, often progressing to Multiple Organ Dysfunction Syndrome (MODS). Even with appropriate surgical and medical management, only approximately half of affected dogs survive to hospital discharge, underscoring the lethal nature of this condition. 2. Etiology and Pathophysiology The etiology and pathophysiology of peritonitis, particularly the infectious variant known as septic peritonitis (SP), describe a cascade in which localized abdominal contamination progresses toward systemic inflammatory crisis. Across studies in dogs, this transition is consistently associated with high morbidity, rapid deterioration, and the need for aggressive diagnostic and surgical intervention. 2.1 Overview of Pathogenesis Septic peritonitis is considered a complex, life-threatening condition, initiated by microbial contamination of the peritoneal cavity and sustained by a dysregulated inflammatory response requiring urgent perioperative management (Mueller et al., 2001). Microbial Contamination of a Sterile Space The entry of bacterial or fungal pathogens into the previously sterile peritoneal cavity represents the defining initiating event. Most common pathogens:Gram-negative organisms, particularly Escherichia coli, are the predominant cause of abdominal sepsis in dogs (Costello et al., 2004). Culture patterns:In one study of canine SP, 39 percent of abdominal effusion cultures yielded only gram-negative bacteria, 28 percent only gram-positive, and 33 percent yielded both, demonstrating the polymicrobial nature of abdominal contamination (Mueller et al., 2001). Sources of Contamination Peritoneal contamination may arise through multiple mechanisms: Surgical Dehiscence The most common cause of SP in dogs in a retrospective study (43 dogs) was dehiscence of an enterotomy or enterectomy site, indicating failure of previous surgical repair (Costello et al., 2004). Ulcer Perforation (NSAID-Induced) Colonic perforation resulting in generalized septic peritonitis has been linked to nonsteroidal anti-inflammatory drug (NSAID) administration, notably meloxicam. Histopathology typically shows full-thickness ulceration, inflammatory infiltration, and perforation with thrombosed vessels at ulcerated sites (Kine et al., 2019). Parasite-Induced Rupture and Ischemia Acute mesenteric ischemia-like syndrome due to suspected Spirocerca lupi aberrant migration causes severe mesenteric vascular thrombosis, intraluminal parasite larvae, and segmental intestinal necrosis. All dogs in one case series ultimately developed septic peritonitis as a result (Lerman et al., 2019). Other Septic Sources Reported infectious causes of systemic sepsis in small animals include: generalized septic peritonitis, pneumonia, pyometra, septic bile peritonitis, and necrotising fasciitis (DeClue et al., 2011). Inflammatory Cascade and Systemic Crisis Once microbial contamination occurs, a pronounced inflammatory cascade leads to: exudation and vascular leakage, accumulation of protein-rich abdominal effusion, endotoxin-driven vascular instability, and systemic toxemia. Sepsis develops when this inflammatory response becomes dysregulated, producing an imbalance between pro- and anti-inflammatory mediators (Culp et al., 2009). The resulting pro-inflammatory shift damages tissues, promotes coagulopathy, disrupts perfusion, and accelerates multiple organ dysfunction syndrome (MODS). Septic shock is
