Bartonella henselae: An Infectious Pathogen among Cats
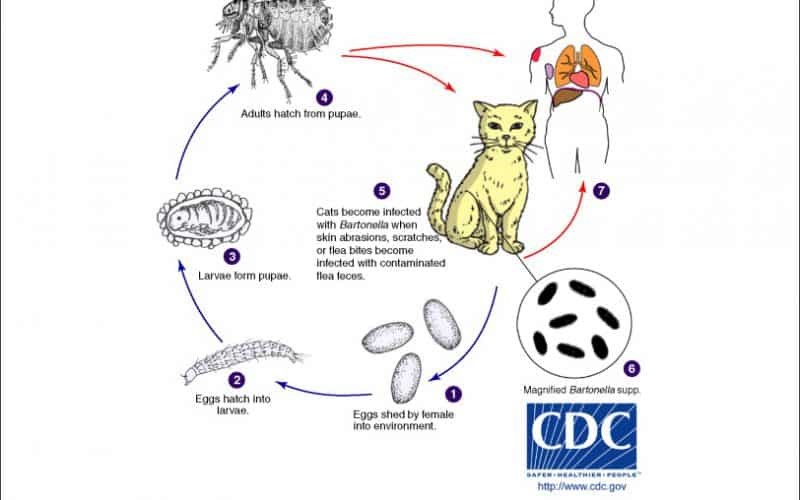
Maigan Espinili Maruquin I. Characteristics / Epidemiology The Bartonella spp. have wide distribution worldwide wherein antibody prevalence in cats which ranged from 8–53% was recorded in Europe (Pennisi, Marsilio et al. 2013, Zangwill 2013) while approximately 5-80% of cats worldwide were recorded of serological evidence on exposure to this bacteria (Guptill 2012). They cause wide range of clinical syndromes depending on the infecting species and immune status of the infected (Zangwill 2013). The Bartonella are small and fastidious Gram-negative bacteria which are transmitted by arthropods and infect wide range of hosts including: human, rodents, rabbits, felids, canids, ruminants. However, cats are the primary mammalian reservoir and vector for transmission (Guptill 2012). The B. henselae is known as a common species to both cats and humans. This species also cause Cat Scratch Disease (CSD) to people. It is naturally transmitted between cats by the flea itself, Ctenocephalides felis felis, or the flea feces. The Bartonella stays in the red blood cells of infected cats and ingested by flea (Chomel, Kasten et al. 1996, Pennisi, Marsilio et al. 2013). While Bartonella persists in the environment in the flea faeces, it also amplifies the infection in the flea hindgut (Finkelstein, Brown et al. 2002). The feces of a contaminated flea, which are deposited in the skin, ends up under the cat’s claw from self- scratching (Chomel, Kasten et al. 1996, Pennisi, Marsilio et al. 2013). Moreover, the tick bites may also transmit B. henselae to humans (Lucey, Dolan et al. 1992, Klotz, Ianas et al. 2011, Biancardi and Curi 2014). While B. henselae are the most commonly detected Bartonella infection in cats and approximately 10% B. clarridgeiae, other species were reported much less commonly. However, the prevalence of different genotypes of B henselae were recorded from regional differences, and are not limited to domestic cats (Guptill 2012). Fig. 01. The Life Cycle of Bartonella spp. (https://www.northcarolinahealthnews.org/2016/12/19/north-carolina-ranks-as-high-risk-zone-for-cat-scratch-disease/) II. Pathogenesis/ Clinical Signs It has been described that B. henselae were transmitted by cat fleas of infected cats to non- infected cats (Chomel, Kasten et al. 1996), or by intradermal inoculation of cats with flea excrement wherein contamination of skin wounds with flea excrement occurs (Finkelstein, Brown et al. 2002). While the infection caused by Bartonella depends on the species and the host immunity (Cunningham and Koehler 2000), infections may cause necrosis with histiocytes, lymphocytes, and giant cells, forming a granuloma for immunocompetent patients (LeBoit PE, 1997) (Biancardi and Curi 2014). Although cats can generate antibody and cell-mediated immune responses against Bartonella infections, the species B. henselae and B. clarridgeiae are commonly chronic and relapsing. On experimentally infected cats, most were clinically normal, while severity relies on the various strains used for inoculation. Abscess were observed on the inoculation sites on cats inoculated intradermally including localized peripheral lymphadenomegaly, short periods of fever (Guptill 2012), mild neurological signs and reproductive failure (Kordick, Brown et al. 1999). On the other hand, cats who received higher doses of B. henselae, despite remaining responsive, showed lethargy, fever, partial anorexia and enlarged lymph nodes (Guptill, Slater et al. 1997, Stützer and Hartmann 2012). Meanwhile, B. henselae naturally infected cats do not show clinical signs (Stützer and Hartmann 2012). However, Bartonella infection was suggested to be associated in chronic gingivostomatitis, but antibodies or organisms’ prevalence in diseased cats were lower (Ueno, Hohdatsu et al. 1996, Glaus, Hofmann-Lehmann et al. 1997, Quimby, Elston et al. 2008, Pennisi, La Camera et al. 2009, Belgard, Truyen et al. 2010, Dowers, Hawley et al. 2010, Namekata, Kasten et al. 2010, Pennisi, Marsilio et al. 2013). Also, there were few cases on B. henselae-associated endocarditis or myocarditis (Chomel, Wey et al. 2003, Chomel, Kasten et al. 2009). The B. henselae, however may be of importance in immune complex diseases in cats wherein a strong correlation between the presence of antibodies against Bartonella species and hyperglobulinaemia was reported (Whittemore, Hawley et al. 2012). III. Diagnosis Having been exposed to fleas, cats with such history, aside from having clinical signs of Batonella infection, shall be tested for possible Bartonella infection (Guptill 2012). Laboratory testing shall be required for feline blood donors owned by immunosuppressed person or when a human with Bartonella-related disease is in the cat’s home (Pennisi, Marsilio et al. 2013). While isolation of the bacterium is considered the gold standard, a positive culture is not confirmatory (Pennisi, Marsilio et al. 2013). The relapsing nature of Bartonella bacteraemia makes the blood culture not so sensitive to diagnose. Thus, this tool is suggested for sick cats with history and clinical presentation of possible infection of Bartonella (Guptill 2012). Therefore, diagnosis is by exclusion, and by assessing the response to therapy (Pennisi, Marsilio et al. 2013). Blood samples, aqueous humour, cerebrospinal fluid or tissues, and several gene targets may be used for PCR (Pennisi, Marsilio et al. 2013). Although standard PCR may be no more sensitive than blood culture, Real Time PCR may have better sensitivity (Valasek and Repa 2005, Kamrani, Parreira et al. 2008, Guptill 2012). The PCR products may be sequenced, which may lead to identification of Bartonella species (Guptill 2012). Serology tests using immunofluorescent antibody (IFAT), enzyme-linked immunosorbent assay (ELISA), and western blot tests are also available (Guptill 2012). However, these are considered to be more useful in exclusion rather than confirmation (Chomel BB, et al., 1995)(Gurfield, Boulouis et al. 2001, Fabbi, De Giuli et al. 2004, Bennett, Gunn-Moore et al. 2011, Pennisi, Marsilio et al. 2013). IV. Treatment and Management There are several drugs that were used to Bartonella infected cats including doxycycline, amoxicillin, amoxicillin–clavulanic acid, enrofloxacin, erythromycin, rifampicin (Greene, McDermott et al. 1996, Regnery, Rooney et al. 1996, Kordick and Breitschwerdt 1997). However, no definite elimination of Bartonella infection in cats by antibiotic treatments was reported but doxycycline may be a good initial antibiotic choice wherein higher doses given for a longer time appears to be more effective (Guptill 2012). A household with immunocompromised people or with children should have their infected cats treated, whether or not they show clinical signs. Whereas,
Feline Pancreatic Lipase (fPL)
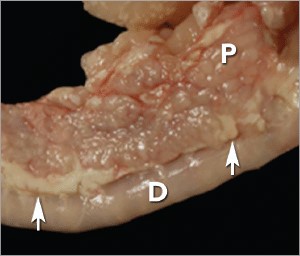
Andy Pachikerl, Ph.D Introduction Pancreatitis appears to be a common disease in cats,1 yet it remains frustratingly difficult to establish a clinical diagnosis with certainty. Clinicians must rely on a combination of compatible clinical findings, serum feline pancreatic lipase (fPL) measurement, and ultrasonographic changes in the pancreas to make an antemortem diagnosis, yet each of these 3 components has limitations. Acute Versus Chronic Pancreatitis Acute pancreatitis is characterized by neutrophilic inflammation, with variable amounts of pancreatic acinar cell and peripancreatic fat necrosis (Figure 1).1 Evidence is mounting that chronic pancreatitis is more common than the acute form, but sonographic and other clinical findings overlap considerably between the 2 forms of disease.1-3 Diagnostic Challenges Use of histopathology as the gold standard for diagnosis has recently been questioned because of the potential for histologic ambiguity.3,4 A seminal paper exploring the prevalence and distribution of feline pancreatic pathologic abnormalities reported that 45% of cats that were apparently healthy at time of death had histologic evidence of pancreatitis.1 The 41 cats in this group included cats with no history of disease that died of trauma, and cats from clinical studies that did not undergo any treatment (control animals). Conversely, multifocal distribution of inflammatory lesions was common in this study, raising the concern that lesions could be missed on biopsy or even necropsy. Prevalence Such considerations help explain the wide range in the reported prevalence of feline pancreatitis, from 0.6% to 67%.3 The prevalence of clinically relevant pancreatitis undoubtedly lies somewhere in between, with acute and chronic pancreatitis suggested to represent opposite points on a disease continuum.2 FIGURE 1. Duodenum (D) and duodenal limb of the pancreas (P) in a cat with acute pancreatitis and necrosis; well-demarcated areas of necrosis are present at the periphery of the pancreas in the peripancreatic adipose tissue(arrows). Courtesy Dr. Arno Wuenschmann, Minnesota Veterinary Diagnostic Laboratory Risk factors No age, sex, or breed predisposition has been recognized in cats with acute pancreatitis, and no relationship has been established with body condition score.3-5 Cats over a wide age range, from kittens to geriatric cats, are affected; cats older than 7 years predominate. In most cases, an underlying cause or instigating event cannot be determined, leading to classification as idiopathic.3 Abdominal trauma, sometimes from high-rise syndrome, is an uncommon cause that is readily identified from the history.6 The pancreas is sensitive to hypotension and ischemia; every effort must be taken to avoid hypotensive episodes under anesthesia. Comorbidities In cats with acute pancreatitis, the frequency of concurrent diseases is as high as 83% (Table 1).2 Pancreatitis complicates the management of some diabetic cats and may induce, for example, diabetic ketoacidosis.7 Anorexia attributable to pancreatitis can be the precipitating cause of hepatic lipidosis.8 The role of intercurrent inflammation in the biliary tract or intestine (also called triaditis) in the pathogenesis of pancreatitis is still uncertain. Roles of Bacteria In one study, culture-independent methods to identify bacteria in sections of the pancreas from cats with pancreatitis detected bacteria in 35% of cases.9 This report renewed speculation about the role of bacteria in the pathogenesis of acute pancreatitis, and the potential role that the common insertion of the pancreatic duct and common bile duct into the duodenal papilla may play in facilitating reflux of enteric bacteria into the “common channel” in cats. Awareness of triaditis may affect the diagnostic evaluation of individual patients. Table 1. Clinical Data from 95 Cats with Acute Pancreatitis (1976—1998; 59% Mortality Rate) & 89 Cats Diagnosed with Acute Pancreatitis (2004—2011; 16% Mortality Rate) PARAMETER HISTORICAL DATA* CATS WITH PANCREATITIS† SURVIVING CATS WITH PANCREATITIS† Number of Cats 95 89 75 ALP elevation 50% 23% 18% ALT elevation 68% 41% 36% Apparent abdominal pain 25% 30% 32% Cholangitis NA 12% 11% Concurrent disease diagnosed NA 69% 68% Dehydration 92% 37% 42% Diabetic ketoacidosis NA 8% 5% Diabetes mellitus NA 11% 12% Fever 7%‡ 26% 11% GGT elevation NA 21% 18% Hepatic lipidosis NA 20% 19% Hyperbilirubinemia 64% 45% 53% Icterus 64% 6% 6% Vomiting 35%—52% 35% 36% ALP = alkaline phosphatase; ALT = alanine aminotransferase; GGT = gamma glutamyl transferase; NA = not available * Summarized from 4 published case series; a total of 56 cats had acute pancreatitis diagnosed at necropsy and 3 by pancreatic biopsy5,8,10,11 † Data obtained from reference12 ‡ 68% of cats were hypothermic DIAGNOSTIC EVALUATION Many cats with pancreatitis have vague, nonspecific clinical signs, which make diagnosis challenging.5 Clinical signs related to common comorbidities, such as anorexia, lethargy, and vomiting, may overlap with, or initially mask, the signs associated with pancreatic disease. Early publications on the clinical characteristics of acute pancreatitis required necropsy as an inclusion criterion, presumably skewing the spectrum of severity of the reported cases.5,8,10,11 Cats with chronic pancreatitis were excluded from these reports. Clinical Findings Table 1 lists common clinical findings in cats from necropsy-based reports and a recent series of 89 cats with acute pancreatitis studied by the authors.12 Note the lower prevalence of most clinical findings in the cats diagnosed clinically rather than from necropsy records. In our evaluation of affected cats, 17% exhibited no signs aside from lethargy and 62% were anorexic. Vomiting occurs inconsistently (35%—52% of cats). Abdominal pain is detected in a minority of cases even when the index of suspicion of pancreatitis is high. About ¼ of cats with pancreatitis have a palpable abdominal mass that may be misdiagnosed as a lesion of another intra-abdominal structure. Laboratory Analyses Hematologic abnormalities in cats with acute pancreatitis are nonspecific; findings may include nonregenerative anemia, hemoconcentration, leukocytosis, or leukopenia. Serum biochemical profile results vary (Table 1). In our acute pancreatitis case series, 33% of cats had no abnormalities in their chemistry results at presentation.12 Serum cholesterol concentrations may be high in up to 72% of cases. Some cases of acute pancreatitis are associated with severe clinical syndromes, such as shock, disseminated intravascular coagulation, and multiorgan failure, that influence some serum parameters, such as albumin, liver enzymes, and coagulation tests. Plasma ionized calcium concentration may be low, and has
Concurrent with T-zone lymphoma and high-grade gastrointestinal cytotoxic T-cell lymphoma in a dog
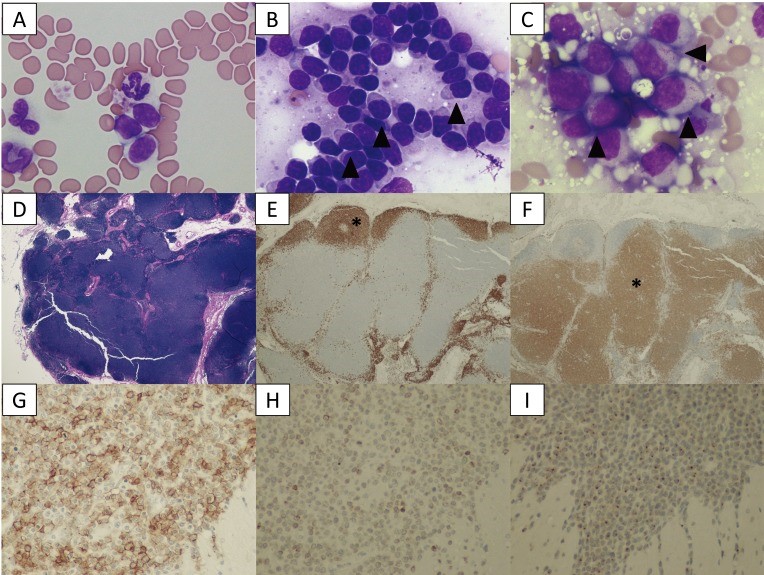
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5402196/ A 9-year-old, spayed female Golden Retriever dog showed lymphocytosis and lymphadenopathy, secondary to suspected chronic lymphocytic leukemia (CLL). Small-to-intermediate lymphocytes were observed from the cytological examination of the right popliteal lymph node via a fine-needle aspirate. The dog was suspected to have a low-grade lymphoma based on the finding of cytology. Also, ultrasonography reveled thickened lesions in the stomach and small intestine. Histopathology of the popliteal lymph node and small intestine revealed a simultaneous presence of T-zone lymphoma (TZL) and high-grade gastrointestinal (GI) cytotoxic T-cell lymphoma. PCR for antigen receptor rearrangements assay suggested that both lymphomas, though both originated in the T-cells, derived from different genes. The dog died 15 days after diagnosis, despite chemotherapy. Fig. 1. A–C: Cytological images on day 1. (A) Peripheral blood smear. Increased numbers of small lymphocytes. (B) Cytology of the popliteal lymph node biopsy. Most lymphocytes are small-to-intermediate, mature lymphocytes. Some lymphocytes show a “hand mirror” type of cytoplasmic extension (arrowhead) (Wright-Giemsa stain, × 400). (C) Slide preparation of tissue from the small intestine. The lymphocytes are intermediate-to-large, immature cells, and some display azurophilic granules in the cytoplasm (LGLs, arrowhead). D–F: Histological images of popliteal lymph node tissue. (D) Hematoxylin and eosin (H&E) staining. (E) The lymphocytes with fading follicular structures are CD20 positive (asterisk). Immunolabeling with anti-CD20, a hematoxylin counterstain. (F) The nodal capsule (CD3 positive) is thinned without the involvement of the perinodal tissue (asterisk). Immunolabeling with anti-CD3, hematoxylin counterstain. G–I: Histological images of the intestinal tissue. All lymphocytes are positive for CD20 (G), CD3 (H) and granzyme B (I). Fig. 2. (A) Transverse ultrasound image on day 1 showing a thickened intestinal wall (approximately 9.0 mm, arrowhead). (B) Post-contrast transverse CT image on day 2 also showing a thickened intestinal wall (arrowheads). The intrathoracic and abdominal lymph nodes are enlarged. Fig. 3. PARR analysis. (A) The peripheral blood sample shows TCRγ gene rearrangement. (B) The intestinal tissue sample also shows TCRγ gene rearrangement. The two tumors demonstrate clonal expansions from different primers.
